Apparently everything now hangs on a vaccine: stockmarkets rise and fall on the mere rumour of how the rumoured development of a particular rumoured COVID-19 vaccine is going; politicians say that normality can’t be resumed until a vaccine is available; people are fed the line that a virus must have a vaccine and that without one the killer virus of death will continue to not kill people, especially those it infects.
Antiviral or vaccine.
The ‘vaccine’ word being blindly banded around by the media is somewhat confusing as it is used incorrectly to refer to either antiviral or vaccine, which are very different.
An antiviral is a drug given to someone either when they are infected with a virus or immediately preceding likely infection. For example, some antivirals for influenza A serotypes must be taken within two days of infection to be effective. The sole function of an antiviral is to attenuate or reduce the target viruss infectability for the here & now. In general antivirals are therapeutic in nature, working only for a short period of time and in effect are a one-time treatment to make you feel less ill.
A vaccine is a drug given to someone before they are infected with a virus. The function of a vaccine is to give an individual long-term – ideally permanent – protection from the virus and in general vaccines are prophylactic in nature, creating active immunity for the future.
How A Vaccine Works.
Vaccines introduce the virus into the body, with the most common forms being live-attenuated and inactivated. Live-attenuated is considered the most effective as it introduces a small quantity of the live but weakened virus so is closest to genuine infection. This also means it generally provides the most effective and long-lasting immunity. Inactivated introduces killed virus particles that can still stimulate an adaptive immune response. It provides less effective immunity that is also shorter-lived and some inactivated viruses require booster shots or – in the case of seasonal or winter flu – a different vaccine every year.
The adaptive immune system is the second stage of the body’s immunoresponse and the one tailored to a specific antigen, in the case of these types of vaccine, the live-attenuated or inactivated pathogen with its specific antigen. However, as a vaccine requires the participation of the adaptive immune system it is reliant upon one of the most important components of the human immune system: immunological memory.
Haven’t We Met Before?
Immunological memory is based upon the concept of primary and secondary infection. Upon getting an viral infection for the first time, while the innate immune system – the first stage of immunoresponse – goes to work to fight the infection, the adaptive system is warming up. Lymphocytes travel to the lymph organs where the specific antigen is presenting to them by antigen-presenting cells. The lymphocytes become lymphoblasts (which would be a wonderful description of lymphocytes having a really good time) and then divide into the two main types of cells that deliver the adaptive immune response: effector B and effector T cells. B cells are the antibodies that target the antigen, by binding to it and rendering it ineffective. Remember antibody cancels antigen.
This process takes 4-7 days to get going – in order to allow the innate immune system the opportunity to do its job first – and is fully operational by 14 days after infection and is already subsiding by 28 days after infection. At this point, the immune system keeps a proportion of the B cells as memory cells. Upon coming into contact with the same antigen again, i.e. secondary infection, in the future, the memory cells immediately recognise it and as they are already active and pathogen-specific, the initial 4-7 day warm up isn’t needed. Instead, they go nuts and start to produce new B cell as well as secrete lots of antigen-specific antibody. This response can be up to 100 times greater than upon primary infection i.e. the number and strength of the antibodies is much, much stronger second time around.
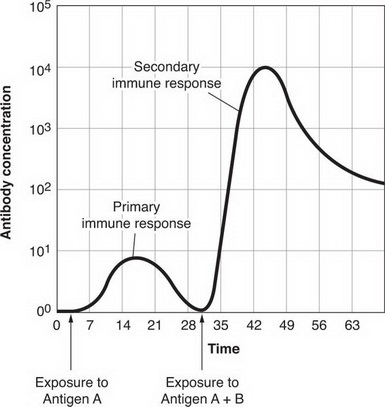
This near immediate and comprehensive secondary response is what immunity is, thereby preventing the virus from gaining hold.
Know your enemy.
Coronaviruses are some of the longest known virus in terms of their RNA and with a genome of 28,936 nucleotides, SARS-CoV-2 is one big boy, as you can see here:
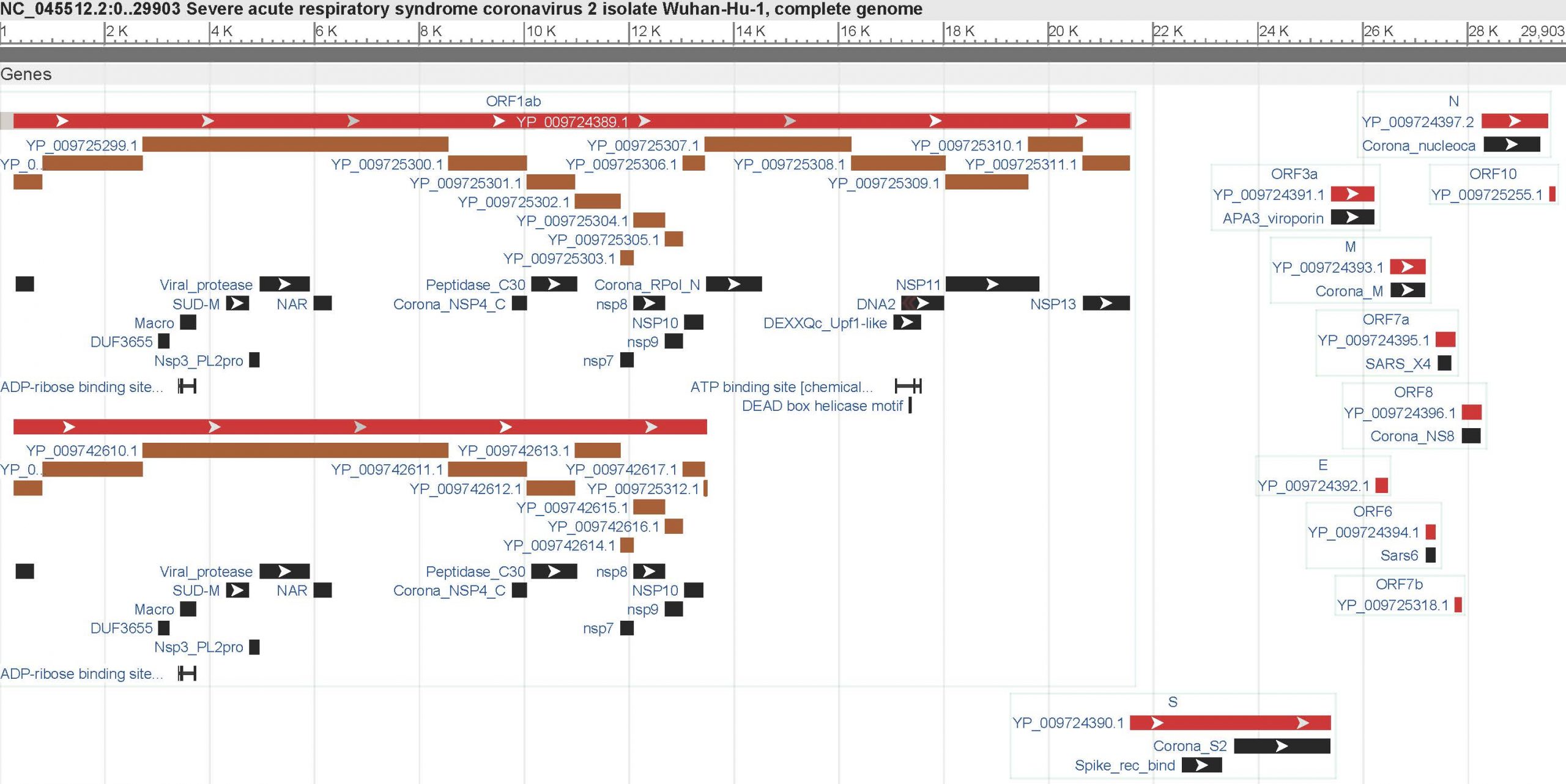
Since its genome was mapped back in January, we have known that its replicase gene is contained in ORF1ab (toward the 5-prime end on the left) and that the virion’s four distinct physical components – its nucleocapsid, envelope, membrane and spike – are encoded toward the 3-prime end on the right. There is the unusual presence of an exoribonuclease in nonstructural protein 14, which acts as a proof-reader during its replication – effectively ensuring that it can accurate & consistently make lots more of itself – and in order for the spike glycoprotein to be activated it requires both furin and transmembrane protease serine 2 (TMPRSS2).
By this point, a vaccine maker should be jumping up & down with glee at all of the obvious points to target. Except of course it isn’t that simple.
Shields Up.
All viruses show a degree of natural selection – as do all humans – and in order to ensure their survival, the mess things up a bit. We have written previously about recombination and reassortment between different viruses but the issue here is where a virus comes up with ways of increasing its virulence through immunoevasion. As a virus, if I know that antibodies are going to bind to my specific antigen then if I change my appearance or make it more difficult for those antibodies to find me and bind to me, I have got more chance of being around for longer. This game of cat & mouse between pathogen and immune system has been going on since the start of existence and is the first obstacle in any therapeutic or prophylactic medication, i.e. it has got to be smarter than the pathogen.
SARS-CoV-2’s spike glycoprotein is how it attaches at the ACE2 receptor binding domain (RBD), goes ‘weapons hot’ and gains cellular entry. So this would naturally be the target for any vaccine, which is why it is naturally the component of the genome that is subject to the highest level of recombination as well as presence of a reasonable level of glycosylation. Up to 40% of the spike protein [Grant et al] is protected by a glycan (carbohydrate) shield that makes it invisible to the antigen-specific antibodies. Think Predator, when Arnold Schwarzenegger covers himself in mud to make himself invisible to the Predator’s infrared thermal imaging. At the same time, the molecular structure of the spike glycoprotein is varied during replication in order to make it harder for the B and T cells to recognise the epitope or epitopes with which their own paratopes have been programmed to bind.
Therefore, while the majority of the spike glycoprotein is not subject to glycan shielding, the high levels of recombination, including antigenic drift that alters the nature of the glycan shield (the glycosylation may increase or decrease proportionately and shift in its location) suggest that any medication targeting the spike protein could be of limited efficacy. However it is noteworthy that the RBD itself is not glycan shielded.
Natural selection through immunoevasion is one of the main reasons why each year’s seasonal or winter flu vaccine is only ever of limited value because influenza A undergoes constant mutation in order to keep the immune system on its toes. The more targeted an antigen-specific antibody, the easier immunoevasion becomes for the pathogen, so you end up with something that is either fairly generic and mildly effective more often or tightly focused and highly effective less often.
Experimental vaccines.
Given the complexities of developing a vaccine that is both safe and effective, it is no surprise that companies spend years, even decades, coming up therapeutic or prophylactic treatments that can be used on humans. Safely and effectively. After all, everything is now about safe, safety, safely and keeping you safe. It is therefore a little surprising, not to mention worrying, that most of the vaccine (not antiviral) development work on COVID-19 is in the area of experimental – not usually a synonym of ‘safe’ – vaccines, in the form of an RNA or mRNA (messenger RNA) vaccine.
An RNA vaccine mimics the RNA genetic sequence of the virus and is designed to perform the same replication on an intracellular basis, i.e. within a human cell as would be the case with the virus itself. However, the replication is controlled as is the target, host cell (so you can in theory avoid the epithelial cells in the lungs being the target as they are with SARS-CoV-2). The desired, targeted cellular entry is enabled through the addition of something called an expression vector, a substance that effectively gets the vaccine where it needs to be.
The relative length of SARS-CoV-2’s genome means there is plenty of space to tack on some other bells & whistles to try and make your vaccine even more effective (would you like to Go Large with that?). One of the main additions is altering the usual nucleotides into nucleosides in order to allow the vaccine to avoid being targeted by the innate immune system before it gets the chance to reach its target cells and – more importantly – unwind its RNA for translation and encoding. This is because the vaccine will immediately be identified as ‘non-self’ by the monocytes and neutrophils of the innate immune system. Another addition is building in a version of an exoribonuclease, this time to oversee an increase in the frequency and/or affinity of replication. In effect this is attempting to make the vaccine more efficient by stimulating the highest numbers of antigen-specific antibodies per unit of vaccine.
A variant – mRNA –is where the genetic sequence is designed to create an antigen-specific antibody, isoform immunoglobulin G (IgG). For a quick refresher on the relevance of immunoglobulins G and M you can read our previous article here. In an mRNA vaccine, the RNA is translated in the cell and encoded to make IgG antibodies that are identical to those that recognise SARS-CoV-2 and therefore the vaccine acts as a top-up to what would be produced by the adaptive immune system.
At this point it is crucial to highlight that there are no approved, functional RNA or mRNA vaccines currently in existence. In development, yes, but not developed to the point of being safe and effective on humans. Bad news if you are a mouse or monkey. Good news if you are not.
This is because the more you genetically engineer RNA by adding stuff to it, the greater the potential for adverse reaction when the proteins come into contact, for example, with existing enzymes that produce an unintended biochemical reaction having never seen each other previously in that particular sequence. Also there is the potential not just for the innate immune system to overreact to what it detects, causing an excessive inflammatory response but also to disrupt the core safety feature of the immune system – the ability to differentiate between self and non-self – which means the body attacks itself, something called autoimmunity.
Remember immunological memory?
Discounting the difficulty in coming up with an effective vaccine, the next factor is that it requires immunological memory in the recipient. Without it, you don’t get the faster, bigger antibody response upon subsequent infection. If someone has a compromised adaptive immune system, for example through frailty, then giving them a vaccine that requires a fully functioning immune system is largely pointless. Higher affinity antibodies won’t be produced by the vaccine and it will be no more effective than the individual’s own immunoresponse.
What has been known since pretty much day one and we have been writing about since April is that the vast majority of individuals who get SARS-CoV-2 are either asymptomatic or suffer mild symptoms (our own when we all deliberately got it back in February-March ranged from ‘bit of a hangover’ to ‘flu-like for a couple of days’). This is a reflection of the fact that the infection is dealt with exclusively by the first-stage innate immune system, as you can read here. While initial lymphocyte activation occurs, the adaptive immune system does not take over from the innate immune and full effector B cell production does not happen.
With the adaptive immune system – and therefore immunological memory – not being needed, a vaccine will not work on these individuals.
Given that COVID-19 deaths (associated and actual) have a comorbidity positive correlation of 0.96 and an average age of 82 years old (source: ONS), a vaccine is unlikely to benefit those at greatest risk.
So the healthy don’t need it and it won’t work on the frail. Just what is the point of a vaccine?
Antivirals – a live wire.
What may be effective – and this depends upon your definition of ‘effective’ – is an antiviral, likely in the form of a monoclonal antibody that targets a specific epitope (or epitopes if bispecific) in the S1 unit of the spike glycoprotein. This is because the RBD remains free of glycan shielding as shielding would render it inactive. Think along the lines of wires in a cable that may be shielded by the plastic casing but where the wires connect, they have to be exposed.
The track record of monoclonal antibodies in neutralising viruses isn’t great: Cosfroviximab and Larcaviximab were deemed ineffective for Ebola, and Diridavumab is 50% effective at best for Influenza A (and that’s according to the company that manufactures it) although Libivirumab may be effective for hepatitis B and Palivizumab is effective for respiratory syncytial virus in infants. Remdesivir – the much-heralded wonder drug for treating COVID-19 – is also an antiviral and while it is effective as a therapeutic for a narrow group with severe symptoms and specific pathogensis of the virus, its track record as an antiviral for previous viruses is not good. It certainly is not an effective antiviral for general use.
This raises the same question as with a vaccine in terms of the efficacy of an antiviral: just how effective might one actually be?
An antiviral may well be the better route to explore when compared with experimental RNA/mRNA vaccine. Perhaps the best outcome that can be hoped for is an antiviral that is 50% effective for those in high risk groups, where it can provide them with passive immunity. This would benefit those with immunosenescence and lacking immunological memory, with an antiviral in effect giving them a second line of defence.