Antiviral Not Vaccine.
The US Food and Drug Administration (FDA) has given full approval to remdesivir for the treatment of COVID-19. Remdesivir has been receiving coverage since the early days of the Great Insanity – often touted as a wonderdrug – and has been in use on a compassionate patient-by-patient basis since late January as a prospective therapeutic treatment for COVID-19. It also gained a much wider audience when President Trump was treated with it earlier this month.
So, at last, a dedicated drug to treat the killer virus of death – everyone should be overjoyed or should they?
The first point to make is that remdesivir is an antiviral and not a vaccine.
We explained the basic differences between a vaccine and an antiviral here earlier this week. In simple terms, an antiviral is a therapeutic medication that is designed to reduce the severity of symptoms and level of infection in someone who is already ill.
An international study [Grein et al, 2020] – evaluating patients in Japan, Italy, Austria, France, Germany, Netherlands, Spain, Canada and the USA – evaluated Remdesivir as a therapeutic for a cohort of patients with severe COVID-19 symptoms, specifically pneumonia, who were also either ventilated (mechanical ventilation or extracorporeal membrane oxygenation) or being given oxygen or had an oxygen saturation level of <94% (an individual’s normal saturation range is 94%-99%). Immediately this highlights the narrow group of individuals for whom the antiviral may be effective as well as the focused nature of the therapeutic treatment.
The study showed that following a 10-day course of remdesivir, 68% of patients showed an improvement in their level of oxygen support, i.e. requiring a lesser degree of support or coming off it. There was a positive correlation between the level of oxygen support and improvement, ranging from 100% improvement for those with low oxygen saturation and low-flow oxygen administration to 56% improvement for those with invasive support:
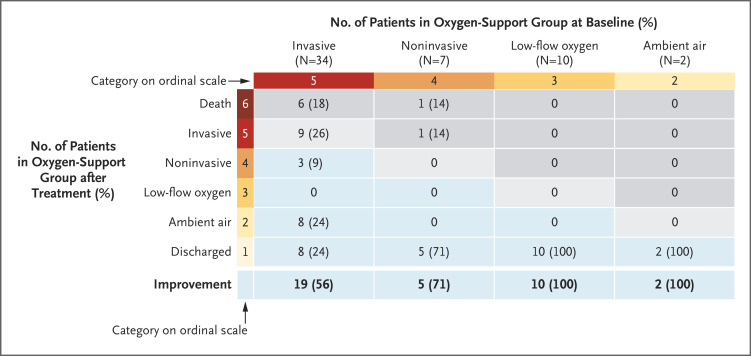
In contrast, 18% of the patients showed a deterioration in their level of oxygen support, i.e. requiring a greater degree of support or going onto it.
Would You Take These Odds?
So, 68% showed improvement, 18% showed deterioration and 14% showed no change or were inconclusive.
If asked, most people would agree to a therapeutic treatment if they had a better than two in three chance of it making them feel less ill.
But if there was a one in five chance of it making them feel more ill, how many would still agree?
Side Effects.
Now factor in the side effects. Remdesivir is known to cause hepatotoxicity, i.e. it is harmful to the liver & its related function. Of course, any medication can have side effects but you would want the benefit to substantially outweigh the drawback(s) before taking any drug. In the study, 60% of the patients reported adverse effects from the treatment and 23% had serious adverse effects.
So there is now a one in five chance of you feeling worse and a one in four chance of you having additional, serious side effects. Still want to take it?
For balance, as ACE2 – SARS-CoV-2’s required target for cellular entry and fusion – is present on cholangiocytes and their derived progenitor cells in the liver, SARS-CoV-2 can target hepatic cells and cause liver damage itself, so liver damage is neither an automatic nor exclusive effect of Remdesivir.
A second, near concomitant study was being undertaken at a hospital in Milan, Italy in the period 23rd February to 20th March. This study [Antinori et al] is important as it was undertaken in a highly-affected region of one of the worst-affected countries (as defined by population-adjusted mortality rate) around the time of peak infection. This study was focused on a similar tightly-focused cohort of patients with severe COVID-19 pneumonia and either mechanical ventilation or <94% oxygen level saturation.
63% of the patients completed the course of remdesivir but all of the other 37% had to discontinue treatment because of adverse side effects. The Italian study differentiated between patients in the hospital’s intensive care ward and its infectious diseases ward. Remdesivir was 88% effective as a therapeutic for those in the infectious diseases ward but less so in the intensive care ward. Of the intensive care ward patients, 22% developed acute kidney injury, with 17% dying of it.
Would you take a drug if there was a one in three chance of you having adverse side effects from it?
Would you take a drug if there was a one in five change of it causing severe side effects leading to death?
The answer to these two questions, as well as the others above, hinges upon just how ill you are. It is the classic quandary used in every Hollywood blockbuster: ‘there is a huge risk if we do this….but if we don’t, something worse might happen instead.’
An Antiviral For A Narrow Group.
For some people, remdesivir is an effective therapeutic treatment. It can reduce the severity of symptoms as well as speed up recovery times. However, it is an effective therapeutic treatment only for those in a very specific group:
a) Those who have current SARS-CoV-2 infection, with the pathogenesis having progressed through cellular binding, entry and active intracellular RNA replication. This is because remdesivir’s antiviral function, once it has itself been activated and gained cellular entry, is to interrupt the RNA replicase enzyme that triggers the copying of the virus’ RNA;
and
b) who have developed severe COVID-19 pneumonia and have respiratory difficultly requiring oxygen support (whether invasive or non-invasive);
and
c) for whom it is not hepatotoxic and who do not develop additional adverse or serious adverse side effects.
Its potential efficacy can perhaps be considered as this: if someone is already at death’s door then a one in five chance of the drug aiding that process doesn’t sound that bad. If someone may die anyway then improving their situation, i.e. therapeutic treatment, is appropriate.
However for everyone else, not only is remdesivir ineffective but with a 60% chance of adverse side effects and 23% chance of serious adverse side effects, the benefit appears not to outweigh the risk.