Newly-Discovered Is Not New.
In early September we first highlighted the incorrect designation of SARS-CoV-2 as a ‘novel’ or ‘new’ coronavirus when it should have been designated ‘newly-discovered’. There is a subtle but fundamental difference: something that is new did not previously exist whereas something that is newly-discovered already existed but not been discovered yet. A new album from your favourite band is different from a newly-discovered band you have just heard for the first time but they have been around for years. So it is with SARS-CoV-2.
By designating it new – as well as ‘Severe’ and ‘Acute’ when it is neither, as per this previous article – everyone was misled into believing that it didn’t exist prior to late 2019 and therefore nobody had it before then. Wrong.
In early September we set out a timeline for SARS-CoV-2’s evolution that goes back to before 2009 and identifies its most recent recombination as being around 2013, meaning it has in all likelihood been in existence for 6-7 years. So why didn’t we know about it before? Simple: it wasn’t virulent enough to come to anyone’s attention and it wasn’t until it became virulent enough to cause symptoms requiring medical attention (or got out, whether by accident or by design, depending upon your view on the source but that’s outside of our scope) and tests were run that its distinct nature was identified. Newly-discovered.
You Can Get It More Than Once – Just Like A Cold Then.
In the stampede to hype up the danger of the killer virus of death that continues to not kill people, especially those it infects, and more recently absolute need to create a vaccine, there has been lots of focus on the little quirks of SARS-CoV-2 that amplify how different it is supposed to be to any other virus in the history of virues and which warrants the government response: these quirks include ‘you can get it more than once’ (gasp), ‘long COVID’ (Gasp!) and ‘antibodies only last a few months (GASP!)’.
If you can get it more than once, it is being dealt with by an innate immune response only. The adaptive immune system is not required. No antigen-specific lymphocyte B or T cell response is needed and so no antigen-specific antibodies are produced. The pathogen isn’t serious enough to trouble stage II, it is dealt with by stage I. Just like the common cold. So the fact you may be able to get it more than once is a good thing. This would explain the seemingly endless rise in ‘new cases’ and government’s desire to get the figure up as high as possible in order to justify its genocidal response. This is why nulceic acid testing is pointless: it tells you nothing other than x number of people have teeny tiny fragments of the viral RNA in their system (which are often viral debris and have been there for months).
A crucial fact from this standpoint is that if a pathogen is dealt with by the innate immune response only, immunological memory is not required and as immunological memory is the central principle of a vaccine, it shows that one will not work. The vaccine will simply be destroyed by the innate response with no B or T cell creation and no respective memory cell conservation.
Long COVID is a figment of Death Secretary Mancock-Shipman-Mengele’s imagination. Another vomitous lie shat out from his fetid mouth. An individual with existing comorbidities who has had moderate to severe COVID-19 symptoms – moderate to severe as a result of their comorbidities – will take longer to recover as their immune system has had harder to work. If you have ever had radiotherapy or chemotherapy you will understand exactly how it feels in the recovery period following a cycle. Read that across to any other illness and that’s ‘long COVID’.
The Contraction Phase.
Antibodies only lasting a few months is exactly how long they are supposed to last. The adaptive immune system starts producing antigen-specific antibodies via immunoglobulin M (IgM) after 4-7 days, superseding them with the more widespread immunoglobulin G after 14 days. By 28 days, the party is over and antibody activity tails off, with the production line stopping and the existing ones dying off because at that point the antibodies are in effect homing torpedoes (‘torpedo in the water, it went live the moment it left the tube’) that are now redundant but still circulating in the blood stream. This is the contraction phase of the immune response and it prevents the risk of a friendly-fire incident where the antibodies mistake ‘self’ for ‘non-self’ and trigger immunopathology. In respect of SARS-CoV-2-specific antibodies, IgM isotypes are gone 56 days and IgG isotypes 80 days after infection [Anding et al, 2020].
So antibodies aren’t meant to last forever, as it would be too dangerous to retain armed weaponry on an accumulative basis: if an individual had had exposure to 30 different pathogens throughout their lifetime, they would have would a full complement of 30 antigen-specific antibodies in circulation, all looking for a target.
Nothing unusual here although it is worth repeating that if the innate immune system is overcoming infection in the vast majority of people who get SARS-CoV-2 it means the pattern recognition receptors on leukocytes and CD56 natural killer cells are recognising the pathogen-associated molecular patterns on the virion. As these are molecular patterns shared by many common pathogens it does not mean that the innate immune system has seen SARS-CoV-2 before, simply that SARS-CoV-2’s genetic make-up is similar enough to some of the patterns. Remember that four other coronaviruses cause the common cold.
Your Serial Killer Friend.
The ‘hello old friend’ bit comes from memory T cells. Naive lymphocytes when shown antigen by professional antigen presenting cells are activated – or programmed – becoming lymphoblasts and then either antigen-specific B or T cells. B cells deal with the humoral response and T cells deal with the cellular response, through CD4 helper cells (TH) and CD8 cytotoxic cells (TC). TC cells hunt down infected host cells, break open the cell’s membrane and effectively inject poison into the cell to destroy the proteins inside (remember that an infected cell is being used as a photocopier by SARS-CoV-2, replicating 16 nonstructural and 4 structural proteins so the T cells’ granzymes have plenty to target), causing apoptosis or the death of the infected cell. TC cells have the ability to go from one infected cell to another and for this reason are often called serial killer cells.
Once they have run out of infected cells to destroy because the infection is over and the contraction phase is underway, TC cells express both CD95 and CD95L – appearing as ‘non-self’ to other TC cells – and kill each other, in a variant of apoptosis known as fratricide. As with B cells, some are conserved and remain in circulation as memory T cells.
Following the SARS-CoV outbreak in 2002, extensive follow-on studies were done on patients post-recovery to monitor their levels of B and T cells. The results are very noteworthy. In one study, memory TC cells were present six years after infection, [Channappanavar et al, 2014], in another, nine to eleven years after infection [Ng et al, 2016] and in another seventeen years after infection [Le Bert et al, 2020].
In the 2020 study, memory T cells from SARS-CoV infection are not only still circulating 17 years after primary infection but can also recognise the nucleocapsid protein – one of its four structural proteins – of SARS-CoV-2. Effectively this means anyone who had SARS-CoV back in 2002-2004 has a level of immunity to SARS-CoV-2. With less than 10,000 identified cases back then, this is only of benefit to a tiny group of people but the startling discovery is from the second group in the study, those who had not had SARS-CoV-2.
T Cells Recognise Three Of SARS-CoV-2 Proteins In Those Who Have Not Previously Had It.
51% of the group who had not previously had SARS-CoV-2 showed a antigen-specific response, with T cells in some recognising and attacking the nucleocapsid structural protein but in a greater proportion of others, recognising and attacking two of the nonstructural proteins, nsp7 & nps13. Both TH and TC cells formed the reactive T cell response.
There is a 100% homology between some of the nonstructural proteins across different species within the same genus, in this case betacoronavirus, with varying levels of homology across different species across different genera, illustrated here:
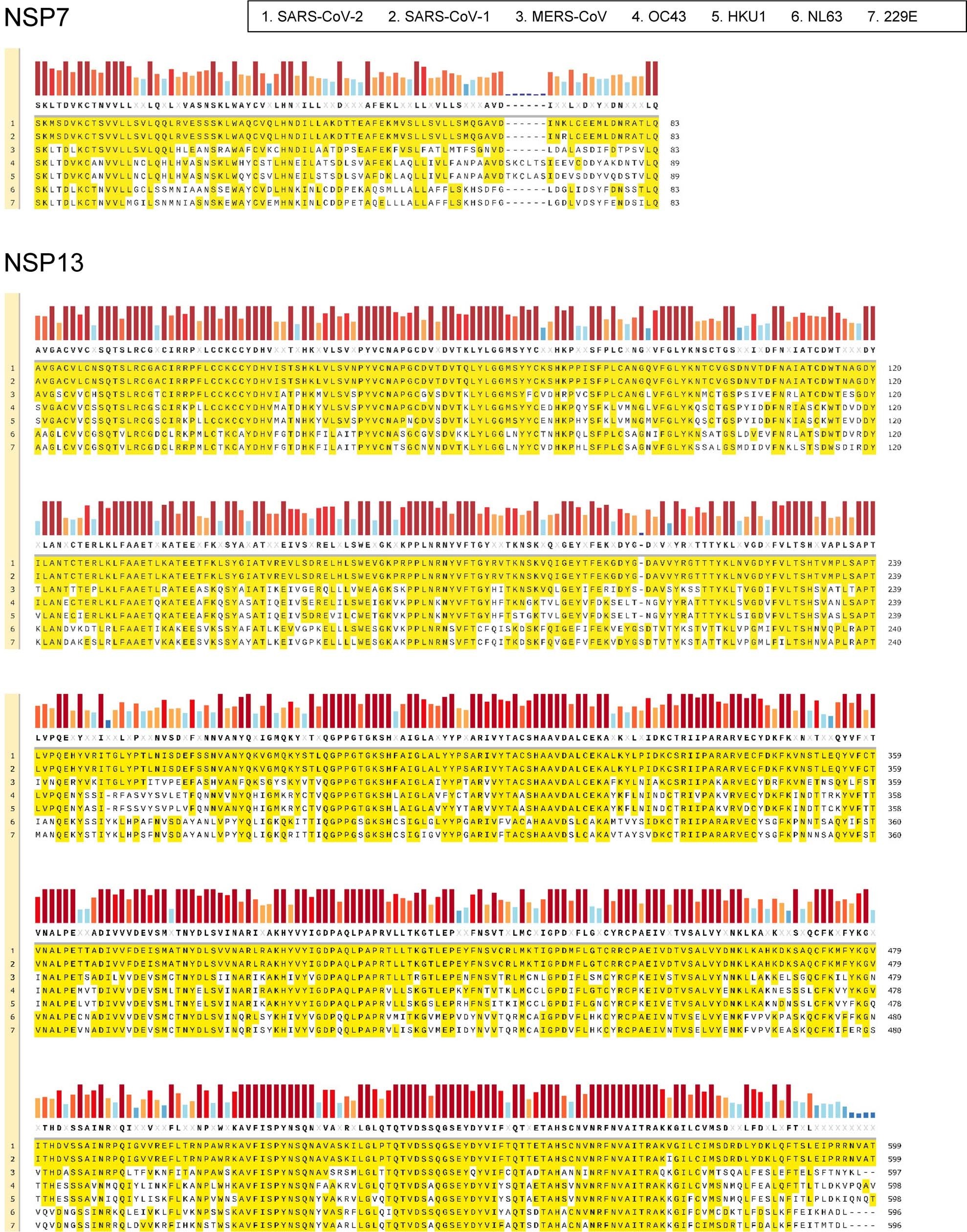
nsp7 & nsp13 alignment across all seven human pathogenic coronaviruses, [Le Bert et al, 2020].
Time for T.
The majority of the attention so far has been on B cells and the related antibody response, usually where the media want to proclaim that ‘antibodies only last a short time’ despite this being their intended duration, as explained above. However, the evidence is building that T cells are the name of the game.
Research shows that not only are SARS-CoV-specific memory T cells still circulating seventeen years after infection but around half of those who have not previously had SARS-CoV-2 possess T cells that can recognise it. The potentially crucial relevance and importance of this is highlighted in a report from Public Health England, the most recent update of which was issued 5 days ago, based upon a study of 2,800 individuals started in June 2020. It states (our emboldening for emphasis):
“About 1/4 of the keyworker population studied had high levels of T cells which recognised SARS-CoV2 in their blood when they joined the study in June. By ‘high levels’ we mean levels similar to those seen in people who have had COVID-19 disease. However, about half the people with high levels of T cells in their blood have not had COVID19“…
“…the cells were probably there because of previous infection with coronaviruses other than SARS-CoV-2.”
T cells are antigen-specific, whether programmed from naive T cells encountering a pathogen upon primary infection or memory T cells encountering a previously-encountered pathogen upon secondary infection, with either event causing a proliferation of T cell creation to fight the infection.
If 50% of the individuals had an active antigen-specific adaptive immune response but had not had COVID-19 then either they had already had SARS-CoV-2 at some much earlier point or SARS-CoV-2 is so similar to another coronavirus that memory T cell proliferation is triggered.
The lymphocyte repetoire can – through V(D)J rearrangement – come up with around 100,000 recombinations to provide antigen-specific recognition, which is very specific. It is not a case of the blue one looking a bit like the purple one. At 50% of individuals, neither is it a rogue memory T cell getting lucky. Those T cells have seen it before. The question is, just what is ‘it’?
‘It’ Is Previous Infection With Another Coronavirus Or Previous SARS-CoV-2 Infection.
We propose two hypotheses as to why a significant proportion of individuals who have not had SARS-CoV-2 have a SARS-CoV-2-specific T cell response.
Hypothesis 1 – SARS-CoV-2 has been around for several years.
Seven coronaviruses are pathogenic to humans: alpha genus HCoV-229E & HCoV-NL63 and beta genus HCoV-HKU1; HCoV-OC43; MERS-CoV; SARS-CoV and SARS-CoV-2.
You can discount SARS-CoV as that stopped being reported in 2004 and was very localised in its nature with distinct symptoms and much higher mortality rate. Likewise MERS-CoV on account of the same two reasons. As we referenced back in August, one of the principal mistakes made early on was the nomenclature of SARS-CoV-2 when it has turned out to be neither severe nor acute but where by making it sound like a re-run of SARS-CoV, it made it out to be far more dangerous than it was or is. So you are left with HCoV-229E; HCoV-NL63; HCoV-HKU1 and HCoV-OC43 – the four coronaviruses that cause the common cold.
Except the common cold is dealt with by the innate immune system so there would not be any antigen-specific memory T cells in existence.
SARS-CoV-2 is classified as a sarbecovirus lineage beta genus coronavirus but we’ve already ruled out SARS-CoV (the only other pathogenic sarbecovirus). You can rule out the alphas as SARS-CoV-2 is a beta genus plus the fact that neither HCoV-229E nor HCoV-NL63 have a furin cleavage site. Rule out the other two beta genus as they are dealt with by the innate immune system – as mentioned above – and you are left with the fact that the proliferated T cells are SARS-CoV-2-specfic and the individuals had previous primary infection, some time before SARS-CoV-2 but before it was designated as such.
That primary infection caused the subsequent conservation of memory T cells which have proliferated upon secondary infection (in 2020), which is the very nature of immunological memory and immunity.
Under Hypothesis 1, SARS-CoV-2 has been around for a number of years, infecting individuals along the way and providing a significant proportion of them with immunity. Those without T cell proliferation dealt with the infection by innate immune response only.
With research proving that memory T cells for a fellow sarbecovirus can last anything from 6 – 17 years (usually limited only by the period of observation) and allowing for the homology between SARS-CoV and SARS-CoV-2 in terms of their nsp7, nsp13 and nucleocapsid sequences, long-term immunity through memory T cells can occur.
Hypothesis 2 – Previous Exposure To Another Coronavirus Can Start Immunity.
Primary infection with one of the main four pathogenic coronaviruses – most likely beta genus HCov-HKU1 or HCoV-OC43, less likely alpha genus HCoV-229E – causes the conservation of memory T cells which then proliferate upon exposure to SARS-CoV-2, as a result of the homology of any/all of the respective nsp7, nsp13 and nucleocapsid proteins.
The level of homology is lower and may be sufficient only to partially arm an adaptive immune response. A full antigen-specific response is not required because by the time the adaptive immune system would be ready to commence its response, the infection has already been overcome by the innate response. However, elements of the virus’ genetic sequence are retained by memory T cells, with both TH & TC cells proliferating upon primary infection with SARS-CoV-2 but which the immunological memory believes to be secondary infection.
This recognition of a similar genetic sequence or pattern is performing the the same function as the pattern recognition receptors in the innate immune response that recognise pathogen-associated molecular patterns.
Under Hypothesis 2, something as simple as catching a cold may kick start immunity against subsequent SARS-CoV-2 infection, by creating memory T cells that have not encountered SARS-CoV-2 but which react to it if they do.
Conclusions.
The comment within the PHE Report – “previous infection with coronaviruses other than SARS-CoV-2” – is only partially correct. T cell proliferation is as a result of previous infection with a coronavirus but that previous infection was either SARS-CoV-2 (before it was identified, in effect pre-winter 2019) or a common cold-causing coronavirus (in our view likely OC-43).
In which case two questions come to mind:
1 How many people had already had it before it was (incorrectly) designated as a novel coronavirus and then designated SARS-CoV-2 in February 2020?
Using just this year’s infections data, it took until only 19th March for an effective level of herd immunity to be achieved in the UK, as we first identified on 14th April. If you roll the start point back several years, how many more people had already had it (under hypothesis 1) or another common cold coronavirus (under hypothesis 2)? More than enough people to make the entire government strategy and response totally unnecessary and pointless.
Instead, government strategy and the government and NHS response is responsible for the deaths of more than 40,495 individuals as a result of lockdown (increasing viral dosage & viral load plus the denial of access to vitamin D synthesis from sunlight outdoors) and failure to treat well-known and treatable conditions of those presenting at hospitals (as well as high viral load from NHS workers). Avoidable and preventable deaths, responsibility for which lies with the deathmongering axis of evil of Emperor Wannbe-Winston Johnson, Death Secretary Mancock-Shipman-Mengele, Chief Manslaughter Officer Saville-Shifty, the Chief Scientology Astrologer and every grovelling, spineless, vile-breath sycophant in government who went along with it because they wanted to bask in the glory of killing their own citizens in the tens of thousands.
2 If immunity can be kick started through contracting a common cold, what exactly is the point of a vaccine?
With memory T cells still in circulation 17 years after recovery from another sarbecovirus, it follows that SARS-CoV-2 immunity may last a similar period without vaccine nor antiviral. That’s where it doesn’t already exist through memory T cell retention from another coronavirus
An antiviral does have utility if it can provide passive immunity to give a second line of defence to those with weakened or compromised immune systems, who do not have the immunological memory to benefit from any vaccine. But most certainly not an RNA/mRNA type experimental vaccine with the truly terrifying potential it has to case autoimmunity, antibody-dependent enhancement and immunopathology.
The generally-considered target for any viral countermeasure to SARS-COV-2 its spike glycoprotein despite this being subject to the highest levels of recombination on the virion, including antigen shift, as well as its dynamic glycan shielding as explained previously here. This makes a vaccine or antiviral more difficult to create and likely to be less effective. However, the fact that coronaviruses have the longest genome of all known RNA viruses highlights a potentially more effective natural countermeasure: the sheer length of the genetic sequence and number of proteins that can be attacked by the granzymes released from TC cells.
The immune system is the most effective countermeasure against SARS-CoV-2.