No Vaccine Required.
Is it possible that the common cold could can provide immunity through memory T cells to a significant proportion of a population? In recent articles we have highlighted various other reasons why a vaccine is not required. This include;
- those not at risk (that’s over 99%) don’t need one and those at risk (that’s under 1%) are unlikely to have immunological memory, so one won’t work.
- an antiviral – not a vaccine – aimed at provided passive immunity to those at risk is likely to be far more effective.
These are not currently approved for any illness anywhere in the world. Hastily-given ’emergency approval’ means cutting corners and essential trials, increasing the probability of autoimmunity, through compromising the most important function of the immune system: the ability to differentiate self from non-self, and immunopathology, especially through antibody-dependent enhancement (ADE).
This list is now joined, as we explained last week, by an even greater and more fundamental reason: the majority of people are gaining immunity through previous exposure to other coronaviruses that cause the common cold.
Memory T Cells.
Over 51% of individuals who had not previously has SARS-CoV-2 have memory T cells that recognise SARS-CoV-2 [Le Bert et al, 2020]. Both memory CD4+ (mainly helper but also regulatory and killer) and CD8+ (serial killer} T cells, upon first time exposure to infected SARS-CoV-2 host cells recognise non structural proteins nsp7 & nsp13 and the structural nucleocapsid protein.
This antigen-specific response without previous antigen exposure is because of the homology of elements of the genetic sequences of coronaviruses in general and those in the beta genus in particular.
A key question would be how one of the two main components of the adaptive immune response – not usually required when dealing with common colds – is being triggered. This is likely down to the relationship between the innate and adaptive components of the immune system and how the complement (also known as the complement cascade) functions as a bridge between them.
The first stage innate immune response doesn’t turn off the moment the second stage adaptive response is turned on. One is warming up as the other is warming down, to ensure maximum protection is maintained and there is no gap in the immune response. Therefore, some B and T cells will have been exposed to the specific antigen and activated. Most of those cells are then killed off but a small number are conserved as memory cells. This is the concept of immunological memory where secondary infection with the same pathogen causes the memory cells to re-activate. As they have higher affinity to the pathogen and require less cell signalling to re-activate, they produce an immune response far quicker and to a greater degree than naive cells upon primary infection [Berard et al, 2002; Swain et al, 2006; Mohrs et al, 2005].
A key reason for this is the minute difference between effector memory T cells (TEM) – localised at the site of infection – and central memory T cells (TCM), in the lymph organs, ready to be sent to the site of infection. TEM cells’ expression of CD62L and CCR7 – required to be able to gain entry to the lymph organs – is downregulated. In effect they are given orders not to go back to base and rearm but to stay put and attack the infection. They also secrete much higher levels of cytokines, including interleukins and interferons, which both target the infected cells and mark the target to call other cells to the location.
We postulate the most important factor is the survival and on-going function of memory T cells through the repeated or persistent presentation of antigen..every time you have a cold.
Persistent Antigen Exposure – A Goldilocks Scenario.
The ‘classic’ concept of immunity is primary infection causing retention of memory cells that are re-activated upon secondary infection where ‘primary’ and ‘secondary’ are with the same pathogen and do not occur on a frequent basis.
The concept of persistency is where memory T cells are exposed to antigen either that they partially-recognise (through homology of the pathogen’s genome) or on a regular basis. Persistent exposure to antigen serves three crucial functions for memory T cells:
- It keeps their numbers up. Memory T cells that do not encounter antigen-specific MHC II reduce in number over time [Kassiotis et al, 2002]. This is because they are in effect redundant and the longer they hang around without being needed, they fewer that are kept on.
- It keeps them primed and on the lookout. They are reminded of their original target [Zinkernagel and Hengartner, 2006] so they don’t forget what it looks like.
- It ensures they are raring to go. The higher affinity to antigen is maintained meaning that memory T cells are more sensitive and will ‘kick off’ at a lower level of antigen presentation than a naive T cell.
While constant exposure to antigen, as is the case with chronic infection, can cause memory cells to weaken [Jelley-Gibbs et al, 2005] and too little can inhibit their survival, just the right amount of regular stimulation can help not only keep them functional but also ensure that an adequate reservoir of them is maintained [Fazileau et al, 2007].
Our most recent article explained that a majority of people who have not had SARS-CoV-2 have a SARS-CoV-2-specific T cell response, which is triggered by the re-activation of memory T cells. This was acknowledged although seemingly ignored in the results data from a study undertaken by Public Health England, Evaluating detection of SARS-CoV-2 antibodies, probably as it was an ancillary observation when the study was observing antibody generation. It is however of immense importance.
Repeated exposure to another common cold-causing beta genus coronavirus – HCoV-HKU1 or HCoV-OC43 – presents sufficient MHC I & II on a persistent basis to memory T cells, which ensures their survival, maintains their function and preserves their memory. Upon any subsequent, primary infection with SARS-CoV-2, the TEM and TCM cells trigger an adaptive immune response, proliferating both CD4 and CD8 T cells.
The common cold has taught the immune system to recognise SARS-CoV-2 and identify it for what it actually is, – a seasonal irritant – and not worry about what it is not, the killer virus of death that continues to not kill people, especially those if infects.
The Virus Continues To Weaken.
Back at the end of August, we highlighted the Active and Closed cases on a global basis which at that point was Active 99% mild/1% serious or critical and Closed 95% recovered/5% deaths. Bearing bear in mind everything that has gone in between, in respect of ‘soaring new cases’, ‘out of control virus’, ‘second wave’, what is the current position?
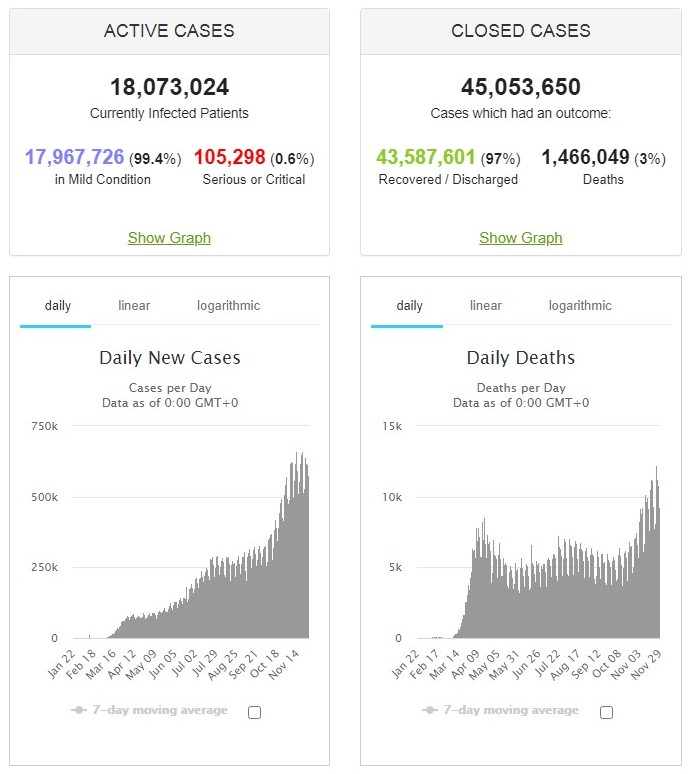
Source: Johns Hopkins Coronavirus Resource Center via Worldmeters, @ 0500UTC 30/11/20.
Active cases are 99.4% mild/0.6% serious or critical and Closed 97% recovered/3% deaths. Yes, over the past three months the virus reduced in severity with both serious/critical infection and death rates falling.
A small proportion of people are at greater risk as is the case for any pathogen, whether SARS-CoV-2, influenza A(H1N1), entamoeba histolytica, norovirus, escherichia coli, enterovirus (all 80+ pathogenic species) or listeria monocytogenes. To put ‘people at greater risk’ in context, 0.05% of people in England have Type 1 Immediate Hypersensitivity – or anaphylactic reaction – to peanuts (source: NHS). The current SARS-Co-2 mortality rate across the entire UK is 0.086% (source: Johns Hopkins Coronavirus Resource Center via Worldmeters, @ 0500UTC 30/11/20).
Shield those at high risk – if they so desire – but as the majority of the population can gain SARS-CoV-2 immunity simply from having had a cold in the past, just what is all this now about?