Learn From The Common Cold.
Pretty much everybody now knows that four coronaviruses cause the common cold: duvinacovirus alphacoronavirus HCoV-229E & setracovirus alphacoronavirus HCoV-NL63 and embecovirus or lineage A betacoronaviruses HCoV-HKU1 and HCoV-OC43. That’s the common cold that you can catch over & over and which is dealt with by the innate immune system only, on account of you being born with pattern recognition receptors, two of which – TLR7 & TLR8 – recognise single-stranded viral RNA.
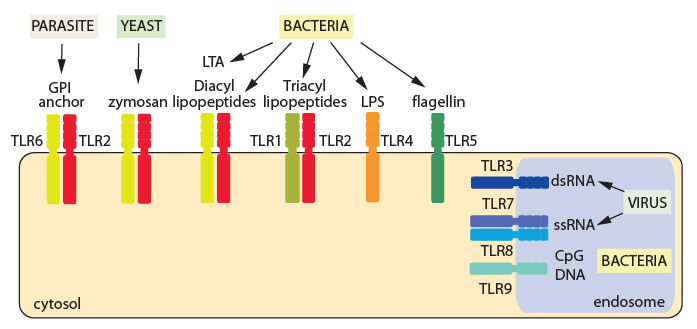
The range of pathogens sensed by toll-like receptors (courtesy: British Society for Immunology).
The government still pushes the ‘stop the spread’ mantra and mega-lie that this is the killer virus of death (the one that continues to not kill people, especially those it infects). So surely repeated exposure must be even more dangerous? No, repeated exposure to a virus can be a good thing.
As we first set out back in early September, sarbecovirus or lineage B betacoronavirus SARS-CoV-2 has been around for several years, most likely recombining with an embecovirus/lineage A betacoronavirus around 2013. While that recombination made it more transmissible and infectious (but not severe or dangerous) it also increased the homology of its genetic sequence. This rendered it more detectable not only to the pattern recognition receptors but to the memory T cells conserved from previous, called-off adaptive immune responses.
Stick Around, We Have Learned Something.
Upon infection with a common cold, the innate immune system mounts the first stage response. As part of this response, a number of the cells that mount the attack on the pathogen are retasked to head to the lymph organs, carrying examples of the pathogen’s antigen with them. There, they present the antigen to naive cells that are then programmed through V(D)J recombination to generate the antigen-specific second stage adaptive immune response. This is in the form of B cells which seek out the antigen in the humoral system (extracellular) and deliver antibodies, and T cells which attack the antigen within infected cells (intracellular).
The process of ‘firing up the Quattro‘ occurs concomitantly to the innate response, so that if the innate immune response is unable to deal with the pathogen, for example if has not seen it before and it evades the pattern recognition receptors, an antigen-specific adaptive response is fully prepared to be deployed. It is this crossover period that is crucial.
If the adaptive immune system is told to stand down, those initial B and T cells already coded with antigen-specific data are not required and so are allowed to die off in a controlled manner called apoptosis. In respect of SARS-CoV-2, B cells expressing IgM isotype are gone less than two months after infection and those expressing IgG isotype within three months [Anding et al, 2020]. The T cells however stick around for much longer as memory T cells, with studies from original SARS-CoV infection showing memory T cells survive anything from six years after infection [Channappanavar et al, 2014] to eleven years [Hg et al, 2016] to seventeen years [Le Bert et al, 2020].
Persistent Antigen Exposure.
Memory T cells remain constantly in circulation but if they do not encounter antigen for a prolonged period of time, their numbers reduce [Kassiotis et al, 2002]. This is logical as the loss of the B cell receptor and related MHC II expression prevents TH cell activation. Therefore, the less frequent the antigen exposure the weaker the potential adaptive immune response.
If the memory cells are exposed to antigen on a more frequent basis, they are in effect reminded of their purpose in life through showing them their original target antigen [Zinkernagel and Hengartner, 2006]. Their numbers and their strength are maintained.
Tonight’s The Night.
Research has shown that regular exposure to the common cold, while dealt with each time by the innate immune system, is presenting sufficient coronavirus-specific antigen to memory T cells to conserve their numbers. Upon any infection with SARS-CoV-2, the homology between embecovirus/lineage A and sarbecovirus/lineage B peptide chains through either MHC I or MHC II expression is activating memory CD4 TH and CD8 TC cells. TH & TC cells present as both TEM and TCM recognise SARS-CoV-2’s nsp7, nsp13 and N proteins.
Given that memory T cells can proliferate up to 100x more than naive T cells [Whitmire et al, 2008] this means that persistent antigen exposure through regularly having a common cold can provide an individual with an antigen-specific adaptive immune response upon primary infection with SARS-CoV-2.
Even more proof that trying to hide away from the virus (one that continues to attenuate and resemble OC-43) is the wrong course of action. Offer shielding to those in high risks groups – if they want it – but for everyone else, go and catch a cold. That’s if you are one of the unlucky few not to have had SARS-CoV-2 without knowing it since it first appeared back around 2013.