What is Antibody-Dependent Enhancement?
As we have set out previously, it takes 10-15 years to develop a vaccine. This timescale is entirely understandable and justifiable when you consider that the vaccine has to be both effective and safe. Safe includes not causing unacceptable side effects, which include the vaccine or immune system’s response triggering autoimmunity, immunopathology or antibody-dependent enhancement. We have highlighted on several previous occasions – the first time on 23rd November, as well as 30th November and 6th December – the specific issue of antibody-dependent enhancement, i.e. where a vaccine or anti-viral makes the illness worse not better.
Antibody-dependent enhancement occurs in two principal ways:
- antibodies facilitate the increased intracellular take-up of virions, causing greater infectivity and viral replication than would otherwise have occurred through natural infection without antibody-response.
- antigens bind to antibodies forming clumps that, in the case of respiratory viruses such as SARS-CoV-2, obstruct the airway and trigger cytokine storm.
Lee et al. show that the second method can occur with SARS-CoV-2, to the degree that the clumps become complex structures that can lead to acute respiratory distress syndrome (ARDS). This is supported by Ye et al. [2017] and Winarwki et al. [2019] in their studies of the effects of antibody-dependent enhancement and antibody-dependent cell-mediated cytotoxicity (ADCC) in relation to influenza A vaccines.
The problem arises when you have either non-neutralising antibodies or neutralising antibodies at ineffectively low levels. Rather than helping with viral clearance, which would normally be the case with antibodies, the antibodies’ Fc domain incorrectly signals ‘help’ and sets off a localised innate immune response at the site of infection. This includes the release of cytokines, which cause inflammation and while this is a natural and automatic part of the innate response, the physical inflammation can make breathing harder. The ‘SOS’ nature of cytokine release also attracts more antibodies and other cells within the innate response, which can become self-fuelling.
Antibody-Dependent Enhancement In Relation To SARS-CoV-2.
It had already been established that ADE was relevant to coronaviruses in general [Gralinski et al., 2018] as well as disease severity for SARS-CoV [Ho et al., 2005; Bolles et al., 2011; Tseng et al., 2012] and COVID-19 in particular [Zhao et al., 2019; Gao et al., 2020; Chao et al., 2020].
The focus of almost all prophylactic and therapeutic treatments currently approved and in development is the structural Spike protein. As we have highlighted previously, this is the obvious target but it is the low-hanging fruit as it ignores the dynamic glycosylation around the S protein, which is SARS-CoV-2’s natural defence mechanism around its antigen. Without the S protein, the antigen can neither bind to target cells nor gain cellular entry to enable viral replication.
It becomes even more interesting when you understand that SARS-CoV-2-specific immunoglobulin G (IgG) isotype antibodies can show low levels of fucosylation in their Fc region [Chakraborty et al., Larsen et al.]. There is an inverse relationship between the level of fucosylation and the affinity of the Fc to bind to receptor FcγRIIIa (also known as CD16a), a receptor that facilitates ADCC. Larsen further shows that the lower the level of fucosylation in IgG antibodies, the greater the disease severity with specific focus upon ARDS.
This highlights the risk of focusing any vaccine or antiviral on the S protein. It also confirms the problem – although not its scale – of concentrating upon IgG istotype as a way of neutralising the S protein rather than other areas of treatment. As part of the Phase I/II trials of ChAdOx1, Ewer et al show stimulation of CD56; CD4 & CD8 T cells after exposure to the test vaccine as well as IgG istotype subclasses 1 & 3:
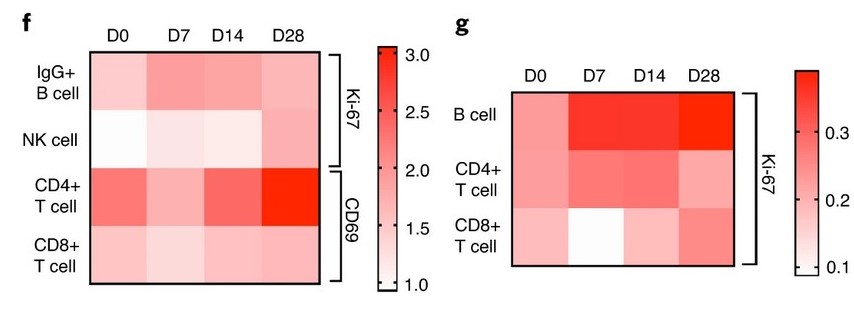
This is all good but firstly the results reinforce the reliance upon IgG and therefore increased risk through antibody Fc afucosylation triggering ADE or ADCC. Secondly it confirms that ChADOx1 does not stimulate immunoglobulin A (IgA), which of course it won’t because it is intramuscular in its delivery. As we have highlighted both last year and earlier this month, ChAdOx1 – as well as mRNA-1273 and others – bypass the upper respiratory tract. Therefore they do not stimulate IgA istotype which is what is required to create sterilising or protective immunity.
Neutralising IgG vs Protective IgA – ChAdOx1 Misses The Action.
Whereas IgG is a neutralising antibody that attacks SARS-CoV-2 once it has bound to ACE2 receptors, IgA is a protective antibody that can stop SARS-CoV-2 from binding in the first place. This is because IgA is abundant in the respiratory tract and in its mucal lining provides a protective barrier against infection.
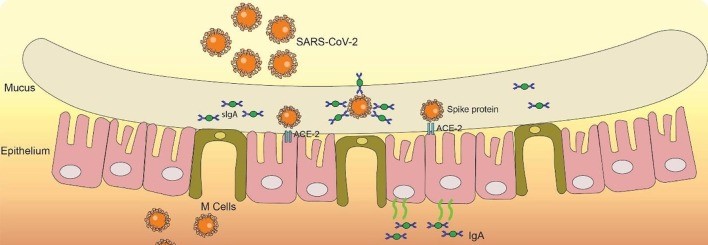
The role of IgA in providing protective immunity from SARS-CoV-2, Chao et al.
In contrast, you can still get and spread SARS-CoV-2 even after being vaccinated with ChAdOx1. An antiviral, at best.
The one-dimensional fixation with the S protein ignores several other areas of weakness and more effective targets to viral countermeasures, notably as we have previously explained nsp14-ExoN’s reliance upon nsp10 for its function and memory T cells from previous common-cold coronavirus infection having a high affinity to nsp7 & nsp13 and structural N protein.
The crucial importance of the upper respiratory tract is shown by Long et al, who identify that higher IgA isotype levels in the upper respiratory tract lead to quicker viral clearance.
The S protein is subject to continuous antigen shift which will make many vaccines ineffective, especially since many will have started out using SD614 before it switched to SG14 (you can read the difference here) and certainly way before S-VoC / N501Y. While studies show a high level of cross-reactivity, focusing solely upon the S protein is unwise as that is the one component of the virion that is subject to regular alteration.
We are not alone in this view, as Peng et al. conclude; “Our data highlight the potential importance of including non-spike proteins in future COVID-19 vaccine design” focused by “Identification of non-spike dominant CD8+ T cell epitopes suggest the potential importance of including of non-spike protein such as NP, M and ORFs into future vaccine design.” This is based upon their study that CD8 T cells show higher affinity for epitopes on the structural M and N proteins than the S protein.
Why Vaccine Development Should Take Time.
Previous vector-based vaccines have form in respect of antibody-dependent enhancement. In a SARS-CoV test vaccine trial using a modified vaccinia Ankara viral vector designed to express the S protein, the IgG expressed to attack the S protein also stimulated immunopathology through increased lung damage [Liu et al, 2019] although to be fair it did reduce viral replication. ‘Good news – your viral load is lower, bad news – you’ve got pulmonary inflammation bordering on ARDS. You’re worse off than had you not been vaccinated’.
Antibody-dependent enhancement can be removed – or at least reduced to a negligible level of risk – but to achieve this takes time. One obvious area to target in mitigating ADE is the Fc region and inhibit its binding with FcγRII/RIIIa. There is also the question of relying upon the assumed interaction in animal testing trials of human Fc domains with Fc receptors of other species used in the testing, such as mice and non-human primates, [Crowley and Ackerman, 2019]. Again, this is why development and the stages of trials require time and therefore have to take time.
An adenovirus vector vaccine trial in 2011 in respect of HIV-1 was halted when a greater number of participants who had received the test vaccine developed the infection than those who received the placebo. ChAdOx1 is an adenovirus vector vaccine.
How Many Red Flags Are Too Many?
The concept of vaccines is wonderful and vaccines have saved tens of millions of lives over the past 220 or so years. Vaccines that are effective and safe. However one cannot ignore these factors:
- Less than twelve months from inception to rushed approval should be a red flag.
- The proven existence of antibody-dependent enhancement in coronaviruses in general and sarbeco / lineage B beta genus coronaviruses in particular should be a bigger red flag.
- The link between IgG Fc fucosylation and S protein glycosylation as well as between Fc afucosylation and FcγRIIIa causing disease severity should be an even bigger red flag.
This last point is made even more relevant by the fact that some IgA isotype subclasses have Fc domains that do not bind to Fc receptors [Kumar et al, 2020].
Perhaps the biggest red flags are these:
- intramuscular & intradermal delivery ignore the upper respiratory tract. This is the first point of contact with SARS-CoV-2. IgA is a protective antibody that can prevent SARS-CoV-2 infection. ChAdOx1 stimulates IgG not IgA and therefore does not provide sterilising or protective immunity.
That’s quite apart from whether S-VoC / N501Y may already have rendered ChAdOx1 ineffective.