B1.1.7 v B1.1.28 v B1.351.
As we have previously explained, SARS-CoV-2 neither thinks nor acts geographically. Yet the misleading designation of SARS-CoV-2 variants on a geographical basis continues to dominate media coverage and government obsession, with the putative ‘third wave’ being down to either the Brazilian variant or the UK variant dependent upon whether you happen to be a Brazilian, European or UK politician.
- B1.1.7(N501Y.v1) is referred to as the UK variant.
- B1.1.28(E484K) is referred to as the Brazilian variant.
- B1.351(N501Y.v2) is referred to as the South African variant.
The first point to highlight is the sub-classification of B1.1.7 and B1.351. Earlier this month we set out that both B1.1.7 and B1.351 share the asparagine to tyrosine substitution in amino acid 501. This means they are both essentially the same, on the basis that SARS-CoV-2 haplotypes appear to be a primary amino acid substitution accompanied by a small number of concomitant amino acid substitutions and related indels and/or single nucleotide polymorphisms. These are likely to support the fidelity of the primary amino acid change and/or increase the variant’s immunoevasion.
Amino Acid Substitutions Are Shared Across Variants.
We highlighted earlier this month that N501Y had been detected in Brazil in April 2020; Australia in June 2020 and both Denmark & the United Kingdom in October 2020, all before it was detected in South Africa in November 2020. The fact that B1.1.7 is now designated B1.1.7(N501Y.v1) confirms this research, as does the application of v2 to B1.351(N501Y.v2) to identify that it did not originate in South Africa. These amino acid substitutions are part of SARS-CoV-2’s natural selection through creating variants with a fitness advantage, as well as its adaptive camouflage through antigen shift.
The fallacy in using geographical names to identify blame variants upon a country is further exposed by the fact that E484K is present in both B1.1.28 and B1.351. Both of these also contain N501Y and a lysine to asparagine (B1.351) and threonine (B1.1.28) substitution at amino acid 417.
In addition, B1.1.298 (the ‘Denmark’ variant) has a tyrosine to phenylalanine substitution at amino acid 453 and B1.1.429 (the ‘US variant’) has a leucine to arginine substitution at amino acid 452.
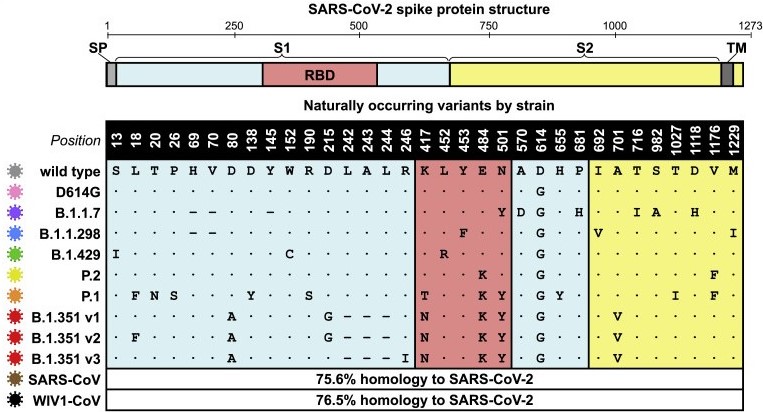
Schematic of SARS-CoV-2 spike protein structure and the mutation landscape of variants, [Garcia-Beltran et al.]
Unsurprisingly for us – and anyone who understands SARS-CoV-2’s pathogenesis and phylogenetic evolution – is that all of these substitutions occur in amino acid positions within the receptor binding domain (RBD) of the spike protein domain. All also occur within the S1 subunit. As S1 shedding is a key factor in increased infectiousness (not infectivity, which remains the same) this is also unsurprising since S1 contributes to fitness advantage.
N501Y is present in all these three main variants. It has nothing to do with geography and this substitution has been around now for eleven months, almost the same amount of time as SD614G, yet another amino acid substitution in the S1 subunit that allowed SARS-CoV-2-SG614 to become the dominant, fixed variant by June 2020. Apart from being what a seasonal irritant does every year, the ‘third wave’ is nothing more than a series of amino acid substitutions, concentrated in the RBD and S1 domains, which have been in existence across the world for just under one year.
All the putative vaccines and antivirals currently approved try to perform clever things with the spike protein but they assume that the spike protein is static and not subject to phylogenetic evolution, whether for fitness advantage or immunoevasion. Therefore their efficacy is questionable. The two exceptions are SaNOtize‘s NORSTM nasal spray, which we evaluated two months ago and Sinovac‘s CoronaVac vaccine candidate, which is a traditional inactivated-pathogen vaccine and therefore presents the entire genome to the immune system rather than just the S protein.
SaNOtize’s nasal spray has the potential to be both prophylactic vaccine and therapeutic antiviral. The difference between the two is set out here.
We have been vehement critics of the obsession with the S protein as a target for viral countermeasures, having identified as early as September 2020 that antigen shift and antigen drift were factors that would likely disrupt any ‘vaccine’ candidate. On 20th October 2020 we concluded; “Perhaps the best outcome that can be hoped for is an antiviral that is 50% effective for those in high risk groups, where it can provide them with passive immunity“.
Spike Protein Variants Dramatically Reduce BNT126b2’s Efficacy.
A comprehensive study published earlier this month validates our concerns. Garcia-Beltran et al. show that E484K; K417N/T and N501Y show high levels of immunoevasion following treatment with BNT126b2 and mRNA-1273. As BNT126b2 is one of the two main antivirals (they are not vaccines) being injected into individuals in the UK, we have focused upon its efficacy rather than mRNA-1273.
BNT126b2 shows a 2.1 fold decrease in neutralising B1.1.7(N501Yv.1) compared to SARS-CoV-2 source genome NC_045512.2. This makes it 52.4% less effective at treating the ‘UK variant’, a somewhat inconvenient truth given it is one of the two antivirals being injected to treat the ‘UK variant’.
For variants containing the E484K substitution – so B1.351 and B1.1.28 as well as B1.1.248(P1) – BNT126b2 shows a 6.7 fold decrease in neutralisation, making it 85.1% less effective. The K417N substitution in B1.351 created an even more significant inhibition of BNT126b2, which shows a 41.2 fold decrease in neutralising B1.351(N501Y.v2).
Inserting the geographical designations solely to reduce confusion around their nomenclature, BNT126b2’s efficacy is reduced by more than 50% against the ‘UK variant’, reduced by over 85% against the ‘Brazilian variant’ and reduced by over 97% against the ‘South African variant’.
This makes a mockery of the hubristic narcissism of Pfizer’s CEO who boasted in November that the results of late stage clinical trials of BNT126b2 were “a great day for humanity“. Considering it is ineffective against variants, ‘a complete waste of time‘ would have been more accurate.
This is further proof that the S protein in general and the S1 subunit in particular are not effective targets for viral countermeasures.
Garcia-Beltran et al. acknowledge that the scope of their study excludes cellular immunity but they correctly reference that “T cell immunity would not be restricted to spike-derived epitopes but also from other more abundant proteins such as nucleocapsid. As such, it would be reasonable to assume that T cell-mediated immunity elicited by infection would remain largely intact for circulating variants including B.1.351.“
This reflects our view, as we first highlighted in November and expanded upon it in December 2020. There is far greater affinity to the structural nucleocapsid protein from CD4 Th & CD8 Tc cells as well as CD56 natural killer cells. The nucleocapsid protein is not subject to antigen shift or immunoevasion and is relatively static. Le Bert et al. [2020] concur in their identification of the greater significance of focusing upon cellular immunity through the T cell response to SARS-CoV-2 infection.
Th cells are also required to assist B cells in the production and subsequent proliferation of immunoglobulin isotypes M and G, as part of the humoral immune response. As BNT126b2 and ChAdOx1 both aim to stimulate IgG then without an effective T cell response, they are far less effective.
Conclusions.
BNT126b2 is significantly less effective against SARS-CoV-2 variants containing N501Y – including B1.1.7 (the ‘UK variant’) – and pretty much useless against those containing E484K and K417N (the ‘Brazilian variant’ and ‘South African variant’).
- The S protein is not an effective target for viral countermeasures.
- The N protein is an effective target for viral countermeasures.
- Any effective viral countermeasure that aims to provide prophylactic protection must stimulate a T cell response, both to provide cellular immunity and to enable humoral immunity.