Treatments Targeting The S Protein Were Already Ineffective.
We have been vehement critics of the miracle ‘wonderdrug’ antivirals (they are not vaccines) rushed onto the market in recent months. In October 2020, we first set out the difference between vaccines and antivirals and highlighted the risks in RNA/mRNA vaccine candidates: these fall into the category of ‘experimental vaccines’, of which none had previously been approved for the treatment of any condition anywhere in the world due to their potential adverse side effects. In December 2020 we expanded our research on why a vaccine was not needed in the first place and that ‘experimental vaccines’ risked disrupting the fundamental function of the immune system: the ability to differentiate ‘self’ from ‘non-self’.
In December 2020 we highlighted SARS-CoV-2’s antigen shift would likely render treatment candidates ineffective due to the dynamic glycan shielding of the structural S protein. For any vaccine – and especially a pandemic vaccine – to be effective, the antigen target must be static. SARS-CoV-2’s S protein is subject to continuous variation, in effect ‘adaptive camouflage’ or ‘stealth mode’ to render it less visible or invisible to antibodies. It is therefore unsurprising to us that two of the main antivirals being injected into individuals in the UK are highly ineffective against SARS-CoV-2 variants:
Pfizer/BioNTech’s BNT126b2 efficacy is
𝗿𝗲𝗱𝘂𝗰𝗲𝗱 𝗯𝘆 𝗺𝗼𝗿𝗲 𝘁𝗵𝗮𝗻 𝟱𝟬% against B1.1.7(N501Y.v1) [‘UK’ variant];
𝗿𝗲𝗱𝘂𝗰𝗲𝗱 𝗯𝘆 𝗺𝗼𝗿𝗲 𝘁𝗵𝗮𝗻 𝟴𝟱% against B1.1.28(E484K)/(P1) [‘Brazilian’ variant];
𝗿𝗲𝗱𝘂𝗰𝗲𝗱 𝗯𝘆 𝗺𝗼𝗿𝗲 𝘁𝗵𝗮𝗻 𝟵𝟳% against B1.351(N501Y.v2) [‘South African’ variant].
Moderna’s mRNA-1273 efficacy is
𝗿𝗲𝗱𝘂𝗰𝗲𝗱 𝗯𝘆 𝗺𝗼𝗿𝗲 𝘁𝗵𝗮𝗻 𝟱𝟲% against B1.1.7(N501Y.v1) [‘UK’ variant];
𝗿𝗲𝗱𝘂𝗰𝗲𝗱 𝗯𝘆 𝗺𝗼𝗿𝗲 𝘁𝗵𝗮𝗻 𝟳𝟳% against B1.1.28(E484K)/(P1) [‘Brazilian’ variant];
𝗿𝗲𝗱𝘂𝗰𝗲𝗱 𝗯𝘆 𝗺𝗼𝗿𝗲 𝘁𝗵𝗮𝗻 𝟵𝟱% against B1.351(N501Y.v2) [‘South African’ variant].
This shows both to be effectively useless at variants containing the E484K-led haplotype, whether with asparagine or threonine substitution at amino acid 417.
Antibody-Dependent Enhancement.
In January 2021, we set out the risk of antibody-dependent enhancement (ADE) as a proven risk in coronaviruses [Ho et al., 2005; Bolles et al., 2011; Tseng et al., 2012; Gralinski et al., 2018; Zhao et al., 2019; Gao et al., 2020; Chao et al., 2020]. The link between IgG Fc fucosylation and FcγRII/III binding, especially polymorphic FcγRIIa/b and FcγRIIa-R131 makes the case for properly researching this risk even more relevant. The capability to develop a treatment candidate that binds to FcγRI only takes time. It cannot be rushed in a few months.
The known, real risk of ADE should dictate treatment candidate development and clinical trials take an appropriate period of time. As we explained in February, true vaccines (i.e. ones that are effective and safe) take 10-15 years to develop, not 10 months.
It is again unsurprising to note that the use of ChAdOx1 (now renamed Vaxzeria) was suspended last month for a time due to it causing thrombosis (blood clots) in otherwise healthy recipients. This should be a ‘never event’.
The putative infectivity of the target illness is not an excuse for otherwise healthy recipients to die of a serious adverse side effect. Just one additional death caused by a vaccine should stop that vaccine’s use. Last week, the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) communicated the risk of blood clots, although for some reason it neglected to mention the risk of anaphylaxis. Subsequently, Public Health England and the NHS updated their guidance for the under-30s, suggesting they have a different ‘vaccine’ to ChAdOx1/Vaxveria. Again, they for some reason neglected to mention the risk of anaphylaxis.
Yesterday (14th April) Denmark stopped the use of ChAdOx1/Vaxzeria, with the Danish Health Authority concluding that; “our overall assessment is there is a real risk of severe side-effects associated with using the Covid-19 vaccine from AstraZeneca“.
At the same time, Johnson & Johnson treatment candidate JNJ-78436735/Ad26.CoV2.S (commonly referred to as Janssen) was being suspended because of concerns about thrombosis and Type I hypersensitive/anaphylactic serious adverse side effects. To an immunobiologist, Type I/II hypersensitivity commands the utmost respect as it is something that is truly terrifying, an extreme immune response that can kill in minutes. For anaphylaxis to be a real risk in a ‘vaccine’ being injected into healthy individuals, who have no need for the ‘vaccine’ in the first place, is beyond belief.
Both Janssen and ChAdOx1/Vaxzeria are adenovirus vector ‘vaccines’ and so it is unsurprising that concerns over the safety of Janssen are also being directed at ChAdOx1 and vice versa. Both Johnson & Johnson and AstraZeneca treatments carry risks of thrombosis and anaphlaxis.
The increasing suspension and withdrawal of both Vaxzeria and Janssen treatments has shifted focus to BNT126b2, with EU President von der Leyen announcing yesterday that an additional 50 million doses of BNT126b2 would now be delivered two quarters earlier (Q2/21 as opposed to Q4/21). To any sane, rational individual, issues surrounding the safety and efficacy of a rushed, corners-cut, safety protocols ignored ‘vaccine’ should be pushing back its mass deployment, not bringing it forward.
Polyethylene Glycol-Induced Anaphylaxis.
If that were not enough red flags, we are now looking into polyethylene glycol (PEG), an ingredient in the lipid nanoparticle coating around the mRNA active element of the treatment.
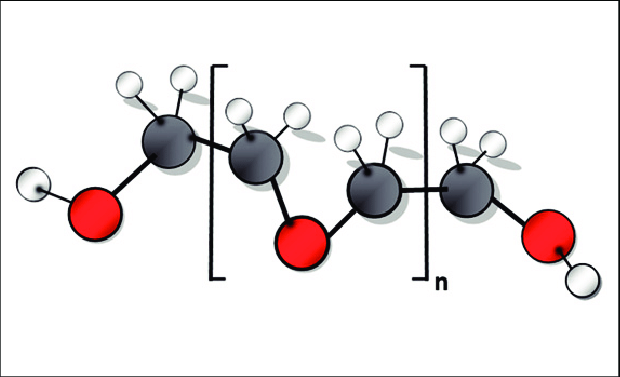
Molecular structure of PEG (image courtesy of ResearchGate).
PEG has never been used previously in any vaccine, in exactly the same way as experimental vaccines had never been approved for the treatment of any condition anywhere in the world before mRNA-1273. Both mRNA-1273 and BNT126b2 use PEG.
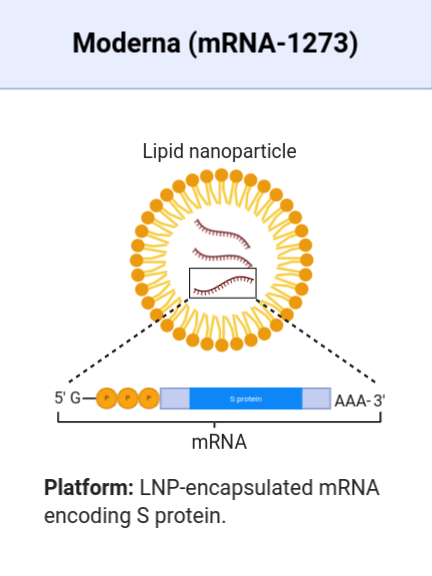
Moderna’s mRNa-1273 vaccine candidate (image courtesy of BioRender).
The relevance is that PEG has been used in other pharmaceutical treatments previously and can cause anaphylaxis. The risk is that individuals who have been treated in the past with drugs that contain PEG can have an increased sensitivity to it through ADE and that its use in a ‘vaccine’ can trigger a hypersensitive reaction. Lai et al. show that 72% of individuals have IgG and IgM isotype antibodies with a high binding affinity to PEG, causing ADE. This is significant as anaphylaxis is usually caused by extreme over-stimulation of IgE isotype only. With IgM being the ‘first-responder’ antibody and IgG the most common, IgM/IgG-mediated anaphylaxis should be a significant concern as it suggests Type II hypersensitivity.
This indicates that ‘vaccine’ candidates containing PEG have clear and real risks of ADE and anaphylaxis. It confirms that the treatment candidates from AstraZeneca; Johnson & Johnson; Moderna and Pfizer/BioNTech all have proven, known severe adverse side effects in the form of thrombosis and anaphylaxis. To substantiate this position, the US Centres For Disease Control and Prevention (CDC) published a study in December 2020 entitled ‘Anaphylaxis Following m-RNA COVID-19 Vaccine Receipt‘. It shows a baseline of anaphylactic reactions to vaccines around 1 per 1million doses. In respect of those who had received COVID-19 ‘vaccines’, there was a 22.1 fold increase in anaphylaxis.
A 22-fold increase in anapylaxis in those who had received an mRNA vaccine is a red flag of almost unparalleled magnitude. Our focus that mRNA had not previously been approved for use on any condition anywhere in the world seems somewhat percipient.
Moderna And Pfizer Knew Of These Risks.
Both Moderna and Pfizer/BioNTech’s clinical trials were fundamentally flawed as they while they found no serious adverse side effects, both sets of clinical trials specifically excluded individuals with any history of anaphylaxis from PEG. In other words, both companies excluded from their trials the very people who were most likely to be at most risk. Interestingly, Pfizer/BioNTech also excluded people with a previous serious adverse reaction to any other vaccine. Again, the very people at greater risk of adverse side effects, those with a history of adverse reactions to a vaccine, were excluded from tests of an experimental vaccine.
The potential risk of PEG has been known for over 20 years, following Szebeni et al.’s pioneering work in 1999 on complement activation-related pseudoallergy. Their work showed that treatments containing nanoparticles encased in PEG can cause the ‘self’/’non-self’ failsafe of the immune system to malfunction, triggering autoimmunity and immunopathology.
Moderna was aware of the risk of PEG-induced anaphylaxis in 2018. In September 2020, BioNTech was already looking at alternatives to PEG as an ingredient in mRNA treatment candidates, concluding that the “PEGylation of nanoparticles can also have substantial disadvantages concerning activity and safety“.
No Vaccine Required.
For 99.6% of the population, no vaccine is required as SARS-CoV-2 infection is dealt with by innate immune response only. The healthy do not need a ‘vaccine’.
An antiviral offering passive immunity to those with advanced immunosenescence and lacking immunological memory would be effective to some degree, as we first posited six months ago, concluding that “perhaps the best outcome that can be hoped for is an antiviral that is 50% effective for those in high risk groups, where it can provide them with passive immunity.“
The scramble to come up with a ‘wonderdrug’ has resulted in established, proven protocols for treatment developments being ignored. Clinical tests have been altered to suit the conflicted agenda of the sponsoring drug company. Proper safety checks have been bypassed. Detailed, time-consuming (it is time-consuming for a valid reason) trials have been cut short. The results are now only just beginning to be seen, as ‘vaccinated’ individuals die of their injection.
Vaxveria, Janssen, mRNA-1273 and BNT126b2 all have fundamental flaws that increase the risk of serious adverse side effects – thrombosis and anaphylaxis – to unacceptable levels. In any other clinical scenario their development would have been discontinued. Instead they are being given to healthy individuals en masse who have absolutely no need of them in the first place, and they are causing serious adverse side effects and death.
As Public Health England and NHS guidance suggests; “You should carefully consider the risk to both you and your family and friends of COVID-19 before making a decision“. SARS-CoV-2 poses little, if any, risk to health in 99.6% of individuals. These ‘vaccines’ pose a far greater risk to your health than SARS-CoV-2.
These antivirals are not safe and are not effective.