Is This Remdesivirmania Redux?
Ivermectin is the subject of increasing coverage and concomitant hysteria around it being another ‘wonderdrug’ for the treatment of SARS-CoV-2. It has been touted as a potential treatment since the days of remdesivir, chloroquine, hydroxychloroquine and lopinavir. We picked apart remdesivir in October 2020, days after it was approved by the US FDA and around the time politicians were standing up in the UK parliament and saying it should be made available on the NHS because it was a ‘wonderdrug’. Our research, using Grein et al. and Antinori et al.’s [2020] studies as baselines, showed that remdesivir might offer therapeutic (antiviral) benefit to a very small cohort only: those with high COVID-19 disease severity and secondary infection with pneumonia and O2Sat requiring oxygen assistance and not at risk of hepatoxicity. In this cohort, the best outcome was shown to be an improvement of one level in the degree of oxygen assistance. 17% of those in intensive care units given remdesivir died of acute kidney injury, likely where remdesivir rather than SARS-CoV-2 was the causative agent. One month later, the WHO agreed with us and recommended it should not be administered, on the basis it was ineffective and there was “no evidence” it reduced mortality.
Ivermectin is a broad-spectrum antiviral used principally for the treatment of infection by parasitic pathogen. It is administered orally for invasive infection and as a cream for external infection. It is generally accepted to be a ‘safe’ medication for parasitic pathogens, having been in use for 40 years as very much an everyday treatment for common parasitic infections.
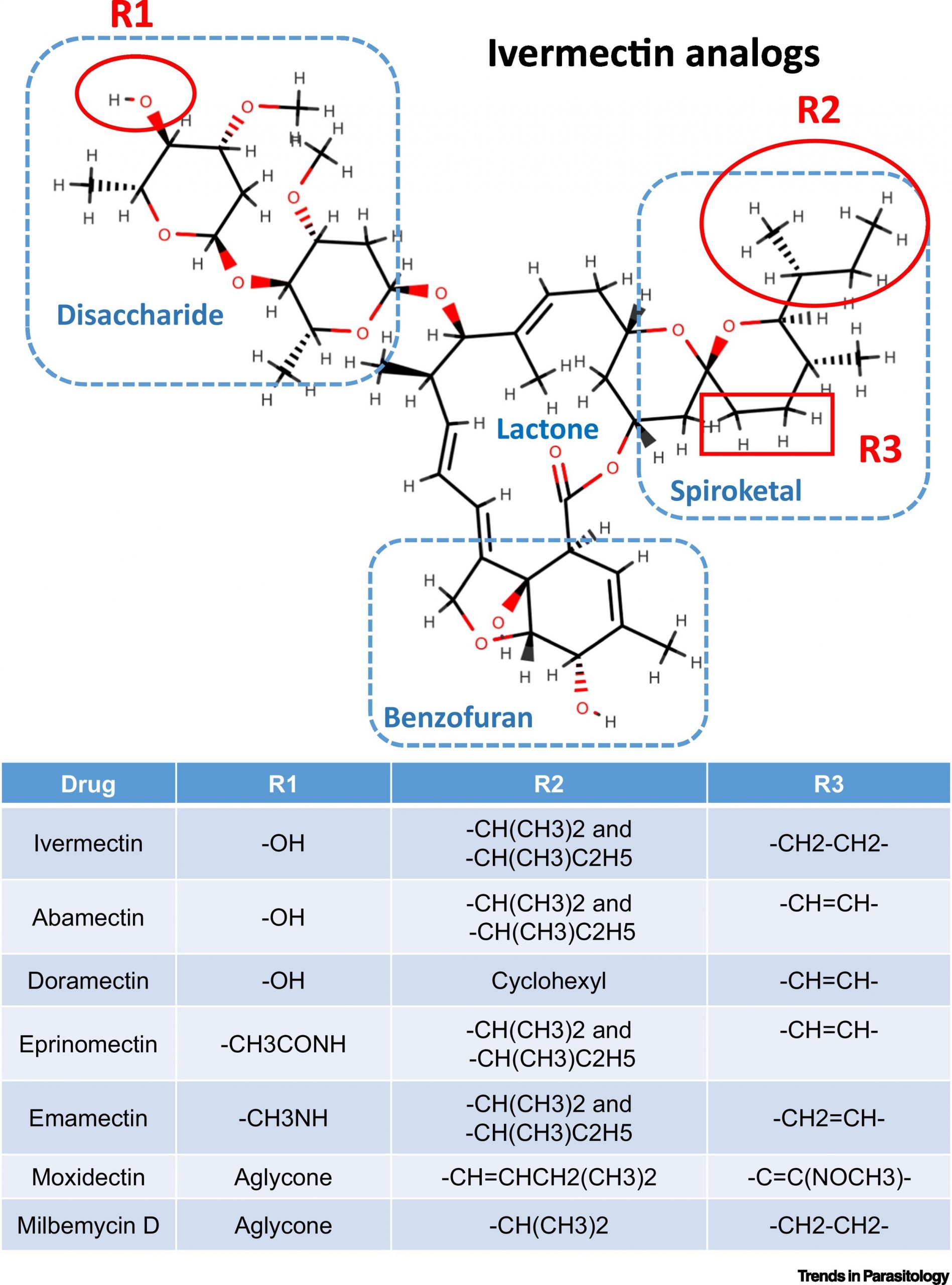
Ivermectin and analogs, image courtesy of cell.com
Disruption And Termination Of Viral Replication.
Its recent rise to prominence is due to it showing high efficacy in disrupting SARS-CoV-2 replication. Upon infection of a host cell, the viral RNA is unpacked into the two polyprotein chains, pp1a and pp1ab. These are then cleaved into the 16 nonstructural and 4 structural proteins. This cleavage process is overseen by papain-like protease (PLpro) and 3-chymotrypsin-like protease (3CLpro, also known as Mpro as it is the main protease). There is evidence that ivermectin targets PLpro – contained in nsp3 – and possibly 3CLpro – contained in nsp5 – thereby preventing assembly of the replication and transcription complex (RTC), and inhibiting viral replication. We first highlighted the relevance of targeting nonstructural proteins as targets for viral countermeasures in November 2020 (for nsp7 & nsp13), December 2020 (for nsp10) and February 2021 (for nsp5).
Caly et al. [2020] show an approx. 5,000-fold reduction in viral replication 48 hours after oral administration of ivermectin. The relevance here is that SARS-CoV-2 is all about viral load in the upper respiratory tract. All the antivirals currently being injected into individuals in the UK are a) intramuscular delivery and b) target the structural S protein. Therefore they bypass the upper respiratory tract and do not stop an individual from catching or transmitting SARS-CoV-2. They may attenuate disease severity (ignoring their inability to recognise variants, especially those containing the E484K-led haplotype with either asparagine or threonine substitution in amino acid 417) thereby shortening recovery time and reducing hospital admissions.
As it is administered orally, ivermectin is effective in the upper respiratory tract and its ability to inhibit/terminate viral replication reduces viral load. Lower viral load reduces the potential for any subsequent disease progression & severity, as well as speeds up viral clearance. Lower viral load also reduces transmission although with a burst size of 103 and replication cycle of a little over 10 hours, it does not prevent it.
Ivermectin is therefore an interesting treatment candidate as it focuses upon viral replication in the upper respiratory tract. Its focus upon PLpro and Mpro, and capacity to inhibit importin α/β receptors – thereby inhibiting transport of the structural Nucleocapsid protein – are noteworthy.
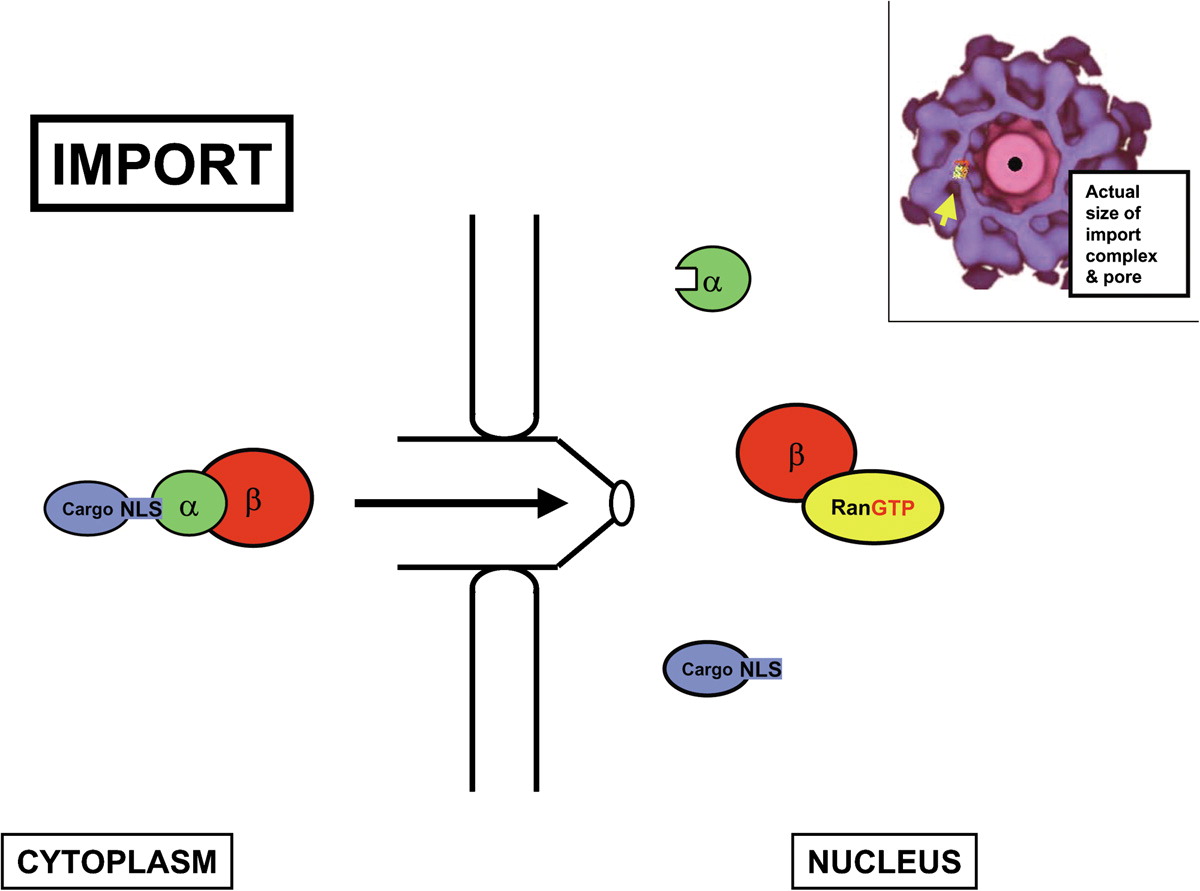
Importin β pathway, image courtesy of cell.com
Ivermectin is also gaining coverage as it is being used in India in conjunction with doxycycline (an antibiotic), mirroring similar ‘wonderdrug’ treatment combinations from 2020 such as hydroxychloroquine/azithromycin and chloroquine/azithromycin. Its use in India is being fermented by social media and word-of-mouth, exacerbated by the lack of availability of perceived ‘vaccines’ that are available in more developed countries. This is unfortunate as India has so far dealt with SARS-CoV-2 far better than most countries, with a mortality rate 2.9-times lower than the worldwide average mortality rate. At 0500UTC today, India ranked 119th out of 219 countries/territories by mortality rate on a populated-adjusted basis (source: Johns Hopkins Coronavirus Resource Center/Worldmeters). This is far more accurate than assessing total deaths – for which it ranks 4th out of 219 – solely because of its population size. Its low mortality rate is as a result of persistent antigen exposure, where individuals’ immune systems are kept more active, resilient and focused through regular low dosage exposure to respiratory viruses, especially those with cross-reactivity of epitopes.
Key Considerations For Use As An Antiviral.
It is important to recognise that ivermectin is not a vaccine. It may be an antiviral, in that is reduces any disease progression & severity; expedite viral clearance and reduces recovery time/hospitalisation.
The first crucial consideration is that when tested on mice, ivermectin has not achieved the intended results as in vitro studies for many of the viruses against which it has reported therapeutic effects. There is also a lack of established clinical trials. We are aware of less than 100 clinical trials of ivermectin in respect of SARS-CoV-2 and many of these are too small sample sizes to be relevant, e.g. Chaccour et al.’s study involved just 24 people (12 target: 12 control) although it is very transparent in highlighting that it is neither statistically relevant nor definitive, rather signalling the need for more detailed study. Cepelowicz-Rajter et al.’s study of 280 patients (173 target: 107 control) with COVID-19 disease severity (principally secondary infection pneumonia) requiring hospitalisation shows a 11.2% reduction (95% CI) in mortality rate in the target group. There was no difference in recovery time between the groups. As the majority of patients in both target and control groups were also given hydroxychloroquine and/or azithromycin, the results are not definitive. Again, the study is open in acknowledging its limitations.
It is worth assessing this reduction in mortality rate with Nogués et al., who show that calcifediol achieves a 57% reduction (95% CI) in mortality rate as well as a 74% (95% CI) reduction in subsequent transfer to intensive care unit post-hospitalisation.
The second crucial consideration is that the established safe blood dosage for ivermectin is in the range 20-80 ng/ml [González-Canga et al., 2008] whereas the levels required to achieve results against SARS-CoV-2 are at least 1,000 ng/ml. While ivermectin requires little metabolism in the kidneys at normal levels, those required for efficacy against SARS-CoV-2 would likely increase the risk of nephrotoxicity. This mirrors the severe adverse side effect of remdesivir, i.e. it causes acute kidney injury and renal failure.
Ivermectin’s use may be appropriate in the early stages of the infection cycle, if it can reduce viral load and therefore reduce transmission. In turn, this would reduce any subsequent disease progression.
Chaccour et al. show that ivermectin administered within 3 days of the onset of symptomatic pyrexia (therefore 5-6 days from primary infection) showed no change in infection 7 days later. However, viral load was shown to be 3-fold lower at day 4 (6-7 days from infection) and 18-fold lower at day 7 (9-10 days from infection). This does indicate significantly lower viral load although in those with mild-moderate viral load, the body would already be moving toward viral clearance in week two of the infection cycle. The study also shows IgG titers were lower at 21 days in those given ivermectin although this is a logical sequela to lower viral load.
Importin Inhibition Is A Double-Edged Sword.
Perhaps the biggest question is how much importin α/β inhibition also inhibits vitamin D pathways. The vitamin D receptor VDR) binds cholecalciferol (vitamin D3) at the beginning of the vitamin D pathway. This VDR:D3 complex is then transported to cellular nuclei by importin-4. As ivermectin inhibits importin then it prevents the synthesis of vitamin D. Vitamin D remains the most effective viral countermeasure both in providing viral immunity (through nitric oxide in the upper respiratory tract) and in preventing disease progression (through calcifediol). Not only does a healthy vitamin D level make an individual more than 10-times more resilient against viral infection but vitamin D deficiency is one of the main factors in increased COVID-19 mortality rates, as we first highlighted in July 2020.
The title of Chaccour et al.’s study perhaps best sums up the potential scope of ivermectin: “…Potential of Ivermectin to Reduce COVID-19 Transmission in low risk, non-severe COVID-19 patients in the first 48 hours after symptoms onset.” In this respect, ivermectin’s use would be similar to that of oseltamivir (Tamiflu), one of the two main antivirals used for influenza A in the UK: its efficacy is greatest when administered within 48 hours of symptoms onset.
A systematic review of ivermectin’s use as an antiviral against a range of RNA & DNA viruses over the past 40+ years [Heidary and Gharebaghi, 2020] suggests that it may be effective as an antiviral in the early stages of infection, both in reducing viral load and reducing transmission. However, they draw attention to the lack of definitive data, both in inconsistent animal trials and a lack of human clinical trials.
Pandey et al. [2020] summarise the position best, concluding “The clinical efficacy and utility of ivermectin in SARS CoV-2 infected patients are unpredictable at this stage”.
Conclusions.
Ivermectin is cheap and has been around for decades, even if for much of its existence it has been in veterinary usage. It is effective as an antiviral against numerous parasitic infections and there is evidence of its varied efficacy against a wide range of RNA and DNA viruses. Ivermectin clearly has potential use as an antiviral, administered in the early stages of the infection cycle, where it may reduce viral load and transmission. Reduce not prevent. It is also active in the upper respiratory tract which is where viral infection and replication occurs. In addition, it seeks to disrupt assembly of the RTC and terminate viral replication by targeting nps3 & nsp5 and the N protein, in contrast to the majority of antivirals (Vaxzeria; BNT126b2; Janssen and mRNA-1273) that target the S protein. In this respect, ivermectin would be effective against SARS-CoV-2 variants and therefore could be a more effective antiviral.
However, the dosage for it to be effective in vitro is at least 50-times higher than the accepted safe level. This would be particularly relevant if it were to be considered to attenuate COVID-19 disease progression & severity in the lower respiratory tract, where the concentrations required for it to be effective could trigger immunopathology. Also, its importin inhibition would likely inhibit other biological mechanisms that are importin-dependent, notably the vitamin D pathway.
Our view is that there are too many parallels with remdesivir: what was initially hyped to be a ‘wonderdrug’ against SARS-CoV-2 was quickly found to be not just ineffective but likely to cause serious adverse side effects in the majority of those to whom it was administered and death in a notable proportion. Ivermectin is targeting the right area – the upper respiratory tract – and components of the SARS-CoV-2 genome that are far less prone to antigen shift and drift, whether for immunoevasion of fitness advantage.
However, in its current format it is not the ‘wonderdrug’ or ‘miracle cure’ that many believe it to be.