Background.
It is difficult to select the most fundamental error relating to SARS-CoV-2: the deliberate confusion of ‘infectiousness’ with ‘infectivity’; the deliberate use of ‘COVID-19’ disease severity when one is referring to ‘SARS-CoV-2’ viral infection, for example ‘rising COVID-19 infections’ when there is no such thing; the use of ‘vaccination’ when the correct description is ‘inoculation’ and the reference to ‘antivirals’ as ‘vaccines’. Another inaccuracy is the use of ‘new’ rather than ‘newly-discovered’, inferring that SARS-CoV-2 is some unique creation that is distinct from its alpha and beta genus coronaviridae relatives and even distinct from every other virus in existence. This is something we first highlighted in September 2020, now over one year ago. Remember that the beta genus phylogenetically evolved from the alpha genus approximately 7,160 years ago [Woo et al., 2020] and therefore the immune system is well-versed in recognising coronaviruses.
In November 2020, we first evaluated SARS-CoV-2 viral immunity through exposure to the other common cold-causing coronaviruses HCoV-NL63; HCoV-229E; HCoV-HKU1 and HCoV-OC43, with Le Bert et al. [2020] showing that memory T cells conserved from having previously had a common cold recognise SARS-CoV-2 antigen as a result of the homology of antigen peptide chains. By the end of November 2020 we had identified that CD4 and CD8 T cells, both TEM and TCM, recognised SARS-CoV-2 antigen in those who had not previously been infected.
Persistent Antigen Exposure – Trained Immunity In The Innate Response.
Using Kassiotis et al. [2002] and Zinkernagel and Hengartner [2006] as the baseline, we then came up with the theory of persistent antigen exposure. This has subsequently become one of the main focuses of our work. Within the innate immune response, it is known as trained innate immunity. This is where regular exposure to various pathogens not only ensures a strong initial immune response – remember SARS-CoV-2 is all about viral load in the upper respiratory tract and a strong innate response prevents accumulation of high viral load – but also cross-reactivity, which is where the innate response to the antigen of pathogen A is also effective against pathogen B, C and so on.
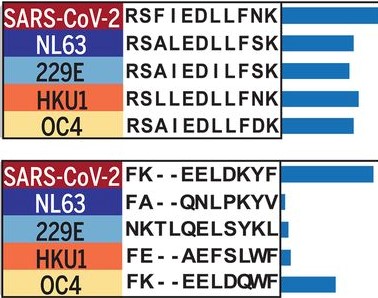
Cross-reactivity of epitopes across coronaviruses, Shrock et al., [2020].
Our work was validated in March 2021 by the University of Glasgow Centre for Virus Research’s study that showed exposure to common cold-causing rhinoviruses provides SARS-CoV-2 immunity (both coronaviridae and picornaviridae families are single-stranded positive-sense RNA viruses), throuugh upregulation of myxovirus resistance protein 1 and therefore interferon signalling.
Trained innate immunity is why the COVID-19 mortality rate in India (as of 0500UTC today, source: Johns Hopkins Coronavirus Resource Center/Worldmeters) is still 1.95-times 𝘭𝘰𝘸𝘦𝘳 than the worldwide average. In comparison, the UK COVID-19 mortality is 3.21-times 𝘩𝘪𝘨𝘩𝘦𝘳 than the worldwide average. Little, if any, social distancing; poor hygiene and lack of developed healthcare for the majority of its people yet India’s mortality rate is 6.28-times 𝘭𝘰𝘸𝘦𝘳 than the UK’s. This is due to trained innate immunity through persistent antigen exposure. Applying India’s mortality rate to that of the UK would equate to 116,989 𝘧𝘦𝘸𝘦𝘳 UK deaths.
Persistent Antigen Exposure – Memory T Cells In The Adaptive Response.
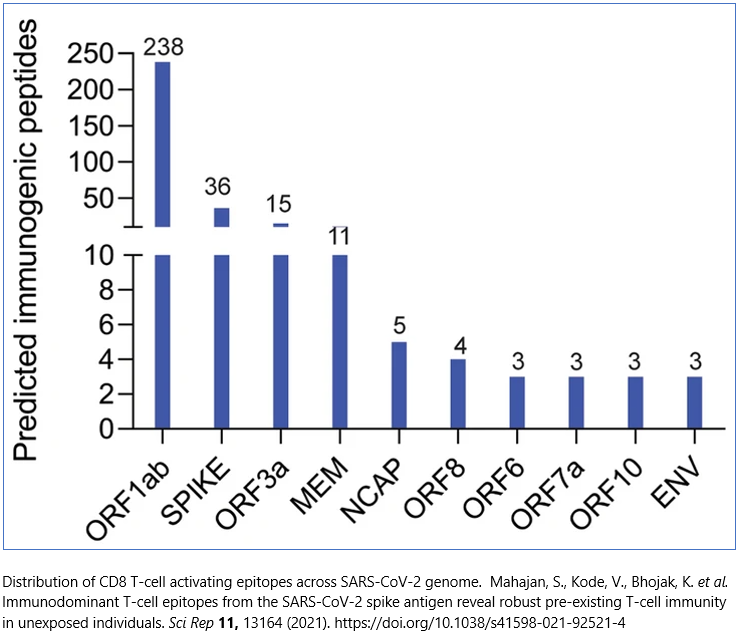
Persistent antigen exposure is becoming more relevant from an adaptive immune response perspective as exposure to winter/seasonal flu – influenza A (H1N1/H3N2) – as well as cytomegalovirus (HCMV, also known as HHV-5) also provides SARS-CoV-2 immunity. As it is the adaptive response, this is again down to the cross-reactivity of memory T cells across flu, HMCV and SARS-CoV-2 epitopes [Mahajan et al.]. These T cells recognise antigen peptide and destroy it, without bothering to ask first if it is A(H1N1), CMV or SARS-CoV-2.
HHV-5 is a species of the human 𝘩𝘦𝘳𝘱𝘦𝘴𝘷𝘪𝘳𝘪𝘥𝘢𝘦 family that includes HHV-1 & 2 (cold sores); HHV-3 (chicken pox); HHV-4 (glandular fever) and HHV-5 (HCMV). HHV viruses are present in 85-95% of all humans. HHV-5 is present in around two-thirds of adults in developed countries and almost 100% of those in developing countries however this is matched by the presence of HCMV-specific antibodies. HCMV has a high degree of latency and will often remain dormant throughout an individual’s entire lifetime, principally on an intracellular basis. After any initial infection, T cells are conserved and in the event of any subsequent flare-up in infection, these memory T cells are re-activated. Crucially, this adaptive immune response lasts a lifetime.
Worryingly for the SARS-CoV-2 antivirals (they are not vaccines) that rely upon replicating the structural spike protein, 89% of T cell cross-reactivity is to SARS-CoV-2 epitopes outside of the spike protein domain:
The list of viruses that can provide immunity to SARS-CoV-2 infection through persistent antigen exposure – both trained innate immunity and memory T cells – now includes the common cold (rhinovirus; duvinacovirus HCoV-229E and setracovirus HCoV-NL63 alpha genus coronaviruses; embecovirus HCoV-HKU1 and HCoV-OC43 beta genus coronaviruses), flu (influenza A) and cytomegalovirus (HCMV/HHV-5).
Persistent antigen exposure within the adaptive immune system means that having had a common cold, seasonal flu or HHV-5 provides long-term, even lifetime, immunity from SARS-CoV-2.
SARS-CoV-2, supposedly the most dangerous virus in the world ever, is a virus against which immunity can be gained simply by previously having had some of the most prevelant, common viruses on earth.