Persistent Antigen Exposure.
We first highlighted persistent antigen exposure back in November 2020, when our research showed that individuals who had not previously had SARS-CoV-2 possessed memory T cells that recognised it, with this recognition likely coming from previous exposure to other common cold-causing coronaviruses. Public Health England that commissioned the report stated (with our emboldening for emphasis);
“About 1/4 of the keyworker population studied had high levels of T cells which recognised SARS-CoV2 in their blood when they joined the study in June. By ‘high levels’ we mean levels similar to those seen in people who have had COVID-19 disease. However, about half the people with high levels of T cells in their blood have not had COVID19“…
“…the cells were probably there because of previous infection with coronaviruses other than SARS-CoV-2.”
Persistent antigen exposure is repeated, low dosage exposure to the antigen of different coronaviruses, especially embecovirus alpha genus coronaviruses HCoV-229E and HCoV-NL63 and lineage A sarbecovirus beta genus coronaviruses HCoV-HKU1 and HCoV-OC43. All of which cause the common cold. Repeated, low dosage means what you would encounter in a work or social environment, such as shopping, in a restaurant or meeting with friends. Going about you daily life and activities without social distancing or non-surgical face coverings.
Pattern recognition receptors TLR7 & TLR8 are programmed to recognise coronavirus antigen. Given that alpha and beta genera coronaviruses diverged around 7,165 years ago [Woo et al, 2020] and coronaviruses first became pathogenic approximately 10,000 years ago at the start of the Holocene, the immune system has had several millennia to hone both its recognition of and response to coronavirus infection. We are all born with an ability to recognise any coronavirus antigen and for the innate immune system to mount an effective response. Whether the species is ‘new’ or ‘newly-discovered‘ the homology of the peptide chains means that the immune system doesn’t differentiate: if it looks like a coronavirus, it will attack it.
Repeated exposure to coronavirus antigen keeps the immune system in peak condition, especially around memory T cell conservation. We explained this in late November and concluded that;
“Persistent exposure to antigen serves three crucial functions for memory T cells:
- It keeps their numbers up. Memory T cells that do not encounter antigen-specific MHC II reduce in number over time [Kassiotis et al., 2002]. This is because they are in effect redundant and the longer they hang around without being needed, they fewer that are kept on.
- It keeps them primed and on the lookout. They are reminded of their original target [Zinkernagel and Hengartner, 2006] so they don’t forget what it looks like.
- It ensures they are raring to go. The higher affinity to antigen is maintained meaning that memory T cells are more sensitive and will ‘kick off’ at a lower level of antigen presentation than a naive T cell.
While constant exposure to antigen, as is the case with chronic infection, can cause memory cells to weaken [Jelley-Gibbs et al., 2005] and too little can inhibit their survival, just the right amount of regular stimulation can help not only keep them functional but also ensure that an adequate reservoir of them is maintained [Fazileau et al., 2007].”
Myxovirus resistance protein 1 increases SARS-CoV-2 immunity.
A study published last week in the Journal of Infectious Diseases, conducted by the University of Glasgow Centre for Virus Research (CVR) shows that rhinoviruses can trigger the same effect. Rhinoviruses are three species of common-cold causing virus within the Picornaviridae family. They are similar to the Coronaviridae family in that both are single-stranded positive-sense RNA viruses, so both are recognised by TLR7 & TLR8 as well as, on a good day, TLR3.
Both bind and undergo their viral replication in the upper respiratory tract and the study shows that co-infection with rhinovirus HRV-A and SARS-CoV-2 inhibited the replication of SARS-CoV-2. This was as a result of HRV-A stimulating interferon production. Interferons are cytokines created in host cells upon invasive infection, which travel to neighbouring cells to warn of the presence of infection and signal the activation of viral countermeasures in those cells. This process is mediated by myxovirus resistance protein 1 (MxA), which both signals interferon production and itself disrupts the virion in the host cell’s cytoplasm, before the virion has the opportunity to commence its replication process.
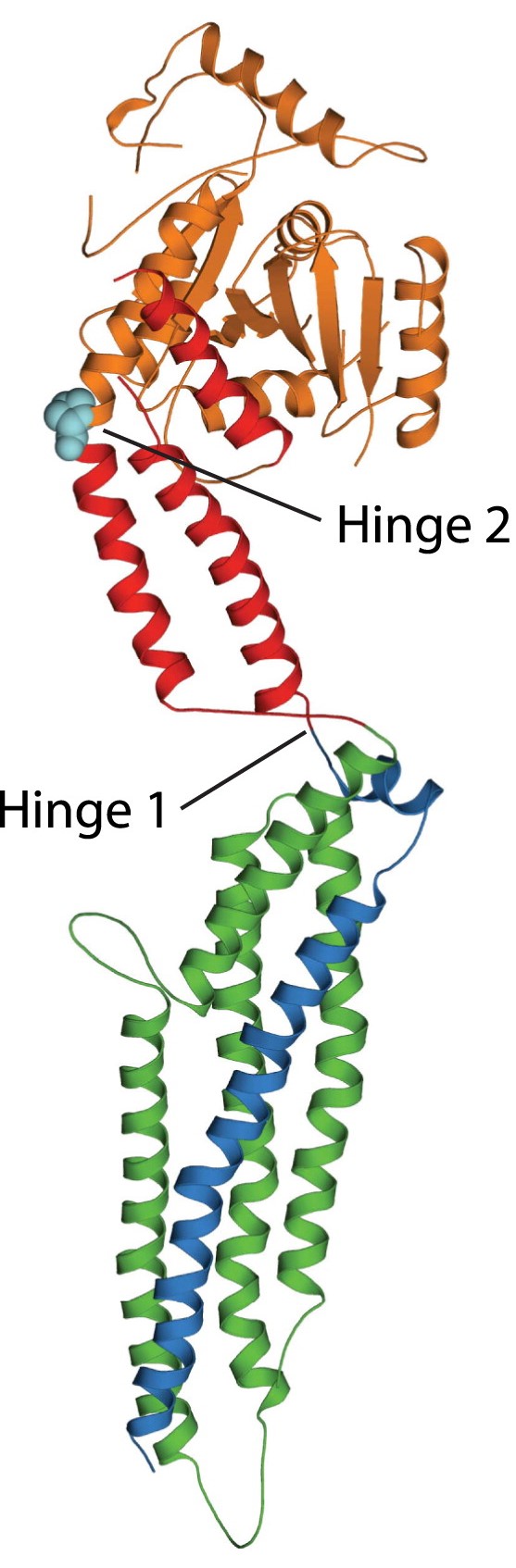
Structure of myxovirus resistance protein 1, courtesy of ScienceDirect.
SARS-CoV-2’s defences include inhibiting interferon production, part of the function of nonstructural proteins nsp1 & nsp3, but this is only effective after the nonstructural proteins have been unpacked from polyprotein pp1a once the replication process is underway within the infected host cell. MxA appears to act like an early warning system and ‘first-strike’ weapon, signalling earlier interferon production and targeting viral replication before nsp1 & nsp3 are unpacked.
The study indicates that SARS-CoV-2 does not stimulate MxA production whereas rhinoviruses do, meaning that either recent previous or simultaneous infection with a common cold-causing rhinovirus increases MxA production. This inhibits SARS-CoV-2’s replication both through MxA targeting the virion before it can begin its viral replication and raising the alarm through interferon stimulation, which causes neighbouring cells to ‘raise shields’ and inhibit the virion’s ability to gain cellular entry.
This is not the same as the memory T cell conservation and activation through repeated exposure to coronavirus antigen but both are examples of how persistent antigen exposure to viruses other than SARS-CoV-2 increases an individual’s immunity to SARS-CoV-2 upon any subsequent primary infection. Persistent antigen exposure is in addition to the recognition of SARS-CoV-2 antigen peptides by TLR7 & TLR8 in the innate immune response and its presentation by MHC I & II to CD4 Th and CD8 Tc cells in the adaptive immune response.
The most effective ‘vaccine’ in existence if your own immune system. The more antigen from similar viruses you come into contact with, the more effective the immune system is if it encounters SARS-CoV-2.
Social distancing and non-surgical face coverings prevent SARS-CoV-2 immunity.