Background.
As early as October 2020 – over ten months ago – we explained why a vaccine was not needed and would not be effective. That’s a vaccine using its correct medical definition, i.e. a prophylactic treatment providing long-term or lifetime protection. All of the four main treatment candidates being injected – Vaxzevria; BNT126b2; mRNA-1273 and Janssen are antivirals. You can read the differences between a vaccine and an antiviral here.
At the time, our view was this;
“Perhaps the best outcome that can be hoped for is an antiviral that is 50% effective for those in high risk groups, where it can provide them with passive immunity. This would benefit those with immunosenescence and lacking immunological memory, with an antiviral in effect giving them a second line of defence.”
A view that remains and one that has been proven correct with the combination of the impact of neutralisation escape variants (which we first identified in December 2020) and – increasingly – the risks posed by experimental vaccines (which we also first identified in December 2020). We picked up on the potential risk caused by antibody-dependent enhancement (ADE) even earlier, in November 2020 and undertook an initial evaluation of ADE in January 2021. There are reasons it takes 10-15 years to develop a vaccine that is effective and safe:
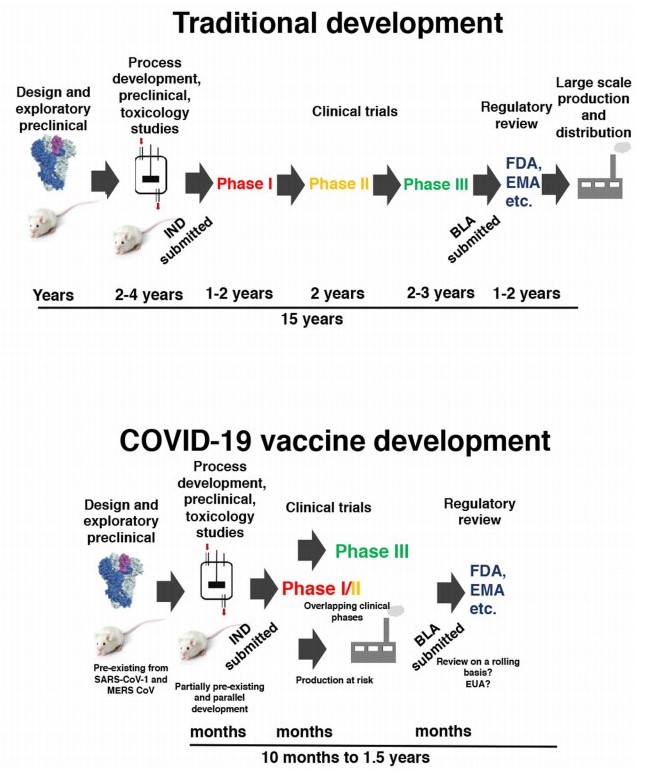
Timeline for standard and rushed COVID-19 vaccine development [Krammer et al, 2020].
In attempts to develop SARS-CoV vaccines, both Bolles et al., [2011] and Tseng et al., [2012] show pulmonary immunopathology from sarbecovirus treatment candidates. Furthermore, ADE is established as a known risk for coronaviridae in general by Ho et al., [2005]; Zhao et al., [2019]; Gao et al., [2020] and Chao et al., [2020].
By mid-April we had isolated two key factors that should be red flags when it comes to treatment candidates, whether antiviral or vaccine: the use of polyethylene glycol (PEG) in the nanoparticle coating and the use of phospholipids as an adjuvant. The elapsed time has expanded the dataset of studies as well as amount of defined further why these are risk factors that have been ignored. Our initial evaluation of polyethylene glycol can be read here.
Polyethylene Glycol And Type II Hypersensitivity.
By way of a brief update, PEG is generally considered to be non-biodegradable, meaning that it cannot be cleared from the body naturally. Lai et al., [2016] show that the majority of treatment-naive individuals (i.e. those who have not previously received any pharmaceutical treatment containing PEG) have naturally-occurring IgG and IgM antibodies that show high binding affinity to PEG. Not something you want when PEG cannot be cleared from the body naturally and instead hangs around: this long-term presence of ‘non-self’ unsurprisingly stimulates those IgG and IgM antibodies, causing a persistent state of immunogenicity. Technically it is type II anaphylaxia with COVID-19 mRNA experimental vaccines causing a 22-fold increase in anaphylaxia risk, as the Centers For Disease Control and Prevention identified in December 2020.
It is noteworthy that Biontech, one of the two companies behind BNT126b2, was already looking at alternatives to PEG as a nanocarrier, before BNT126b2 gained its initial approval in 2020., in the form of polysarcosine (pSar). pSar is a derivative of the amino acid glycine and therefore is of natural origin, with sarcosine being expressed in human muscular tissue. Their study observes that pSar offers;
“an improved safety profile after intravenous injection“, meaning that PEG has a lower safety profile;
“a higher protein secretion with a reduced immunostimulatory response was observed when compared to systems based on polyethylene glycol (PEG)”;
“a lower proinflammatory cytokine secretion and reduced complement activation compared to PEGylated LNPs [lipid nanoparticles]”.
It concludes by saying “pSar-based LNPs enable safe and potent delivery of mRNA, thus signifying an excellent basis for the development of PEG-free RNA therapeutics.”
At least they deserve credit for correctly describing BNT126b2 as a therapeutic – therefore antiviral – rather than prophylactic – therefore vaccine – treatment.
So, pSar shows a lower pro-inflammatory response and downregulation of complement activation than PEG, which means the converse is also true: PEG shows higher pro-inflammatory response and upregulation of complement activation. The pro-inflammatory signalling pathways on viral infection can lead to cytokine storm (principally interleukin-6 and tumor necrosis factor alpha) and if not modulated, both systemic inflammatory release syndrome (SIRS) and its counterfunction compensatory anti-inflammatory release syndrome (CARS). Therefore, you would want any treatment candidate and especially a pandemic one – given the number of potential recipients – to use the safest delivery methods possible. You would also expect the same requirement given the increased expression of complement factors C3a and C5a in those with high COVID-19 disease progression and severity. PEG fails on both these requirements.
In September 2020, Biontech itself said; “PEGylation of nanoparticles may also have significant disadvantages concerning activity and safety“.
There are warnings accompanying both BNT126b2 and mRNA-1273 that individuals should be monitored for around 15 minutes after administering the treatment. This assumes type I hypersensitivity that presents within minutes of activation of the immune response, e.g. as with a nut allergy. This makes out that any anaphylaxia risk is based upon a type I response which is incorrect as the risk is type II, which is humoral. This may occur at any point in the future, particularly in the event of any administering of another, unrelated pharmaceutical treatment that happens to contain PEG.
This is the application of the two-hit theory where naturally-occurring antibodies are not pathogenic per se but rather an external stimulus. Administering a ‘vaccine’ containing PEG (remember that PEG has not previously been used in any vaccine and that no mRNA experimental vaccine had previously been approved for any condition anywhere in the world) is the first hit and then subsequent contact at a later date with the same or a different treatment is the second hit. Unless of course someone has in the past taken a PEGylated treatment for an unrelated condition and so has already attained the first hit.
Phospholipid And Antiphospholipid Antibodies.
Antiphospholipid (aPL) antibodies are a group of autoantibodies, i.e. they mistake ‘self’ for ‘non-self’ and target healthy cells or tissue. aPL antibodies bind phospholipids or protein:phospholipid complexes. They principally consist of anti-beta2 glycoprotein 1 (aβ2GP1); anti-cardiolipin (aCL) and lupus anticoagulant (LA) antibodies. While anti-phosphatidylserine/prothrombin (aPS/PT) antibodies may not be categorised as aPL, they are biomarkers of antiphospholipid syndrome (APS, explained below) so they are included.
It is generally accepted that up to 5% of healthy individuals have antiphospholipid antibodies in natural circulation but they are important as increased production of aPL antibodies is prothrombotic, causing both arterial and venous thromboses. These can be anything from a single microvascular thrombosis to large vessel thromboses across multiple organs. Antiphospholipid antibodies can cause antiphospholipid syndrome (APS), which is excessive thrombotic and thromboembolic events. APS is the main cause of acquired (as opposed to inherited) thrombophilia in the under 50s, with arterial thrombosis causing heart attacks and strokes, as well as having an impact on pregnancy loss as aβ2GP1 binds to annexin V protein that is highly expressed in the placental endothelium.
The concern is that mRNA experiment vaccines have been shown to activate coagulation factor with both mRNA and vector vaccines triggering type I interferon-mediated upregulation of antiphospholipid antibodies [Talotta and Robertson, 2021]. It is ironic in the extreme that one of SARS-CoV-2’s defence mechanisms, as we set out in May, is interferon inhibition through evading detection by pattern recognition receptors RIG-1 and MDA5, which would normally upregulate interferon-I signalling.
Mendoza-Pinto 𝘦𝘵 𝘢𝘭. [2018] show aPL antibodies can be stimulated following infection and following vaccination, which is unsurprising since both BNT126b2 and mRNA-1273 use phospholipids in their nanoparticle coating.
Antiphospholipid antibody activation causes disruption in the endothelium and triggers a signalling pathway that includes increased molecular adhesion, including increased expression of ICAM-1 and P-selectin; increased cytokine signalling; creation of neutrophil extracellular traps (NETs) and further activation of coagulation factors, including platelet activation factor. If that were not sufficient, as phospholipids are highly expressed in pulmonary surfactant within the alveoli, aPL binding increases the permeability of the alveolar-capillary barrier through which gas exchange occurs during breathing. The result is respiratory difficulty ranging from dyspnea to exudiative ARDS. Acute respiratory distress syndrome is one of the main symptoms of high COVID-19 disease severity but it can also result from antibody-dependent enhancement, as we set out in January, where antibodies at ineffectively low levels, i.e. the inability to bind effectively to neutralisation escape SARS-CoV-2 variants, form antigen:antibody clumps that in the case of a respiratory virus can obstruct the airway and lead to ARDS. This position remains valid but is compounded now by ARDS also being caused by APS and its most extreme form, catastrophic antiphospholipid syndrome (CAPS).
This creates the situation where ARDS may be caused by two different conditions that may exist simultaneously but where their respective symptoms are very similar, although not their respective biomarkers.
As Espinosa et al. [2001] conclude; “Clinicians should seriously consider these types of vascular injury when evaluating patients with APS who present with dyspnoea, fever, and infiltrates on chest radiography. These conditions might occur as separate entities or may, in fact, form part of ARDS. Therefore, in the presence of these clinical features, clinicians must consider searching and testing for aPL when no other cause can be determined.”
High COVID-19 disease severity means that the infection has spread from the upper to lower respiratory tract and is causing tissue damage to pneumocytes and alveolar macrophages. APS is also expressed heavily in pulmonary dysfunction and thromboembolism. Both are accompanied by pyrexia as part of the immune system’s default response to any infection, which creates a situation where an individual will present with pulmonary symptoms of COVID-19 disease progression that are very similar to antiphospholipid antibody-mediated APS/CAPS.
Antiphospholipid antibodies upregulate the creation of NETs in the extracellular matrix of the endothelium, causing the proliferation of microvascular thromboses [Kapoor et al., 2018; Thålin et al., 2019] that can develop into APS. Zuo et al. [2020] posit that the “production of aPL antibodies potentiates NET formation and BAFF [B-cell activation factor] release.” Maturing B cells may then class switch from first-response IgM to main response IgG isotype. They continue that “the interplay between COVID-19 and humoral immunity is an area that merits further study“, something with which we agree, having argued all along that you do not use the humoral system as the target for any treatment: firstly because SARS-CoV-2 binds and replicates in apical ciliated epithelial cells in the upper respiratory tract (the domain of IgA isotype) and secondly because if you target IgG and IgM isotypes in the endothelium you risk ADE, which is exactly the situation that is now unfolding.
In May we explained that the routine testing upon hospitalisation with COVID-19 disease progression for three proteins – C-reactive protein; procalcitonin and interleukin 6 – would have saved thousands of lives. The cult-like, obsessive focus upon PCR testing ignores these far more relevant tests, which can provide patient-specific, real-time biomarkers of COVID-19 disease progression and severity. To this list one should add aPL antibodies.
APS is an established if not widely-known condition. Argento and DiBenedetto [1998] conclude; “Patients with APS are at an increased risk of widespread vascular thrombosis due to the presence of circulating antiphospholipid antibodies. This case emphasizes the importance of recognizing APS and its potential for multiple organ system involvement”. Testing for aPL antibodies is an effective biomarker for APS.
The Importance Of Beta-2 Glycoprotein 1.
The relevance of aβ2GP1 in particular is significant because of the functions performed by beta-2 glycoprotein 1 (β2GP1). These include maintaining homeostasis and hemostasis, in particular inhibiting coagulation factor, i.e. it prevents clotting. In addition it regulates the immune response and along with C-reactive protein and one other protein, β2GP1 is unique in being able both to up and down regulate complement activation. Given the excessive levels of C3a and C5a complement factors present in high COVID-19 disease severity, β2GP1 is worthy of focus.
Downregulation of β2GP1 as a result of aβ2GP1 binding both increases clotting risk – which is prothrombotic – and increases expression of aPL antibodies. In addition, the loss of control over complement activation may be a reason for the increased expression of C3a and C5a factors. This connects to the increased expression of P-selectin in the endothelium (C5a regulates P-selectin) as part of the aPL-mediated signalling pathway and explains why P-selectin is highly expressed in those with high COVID-19 disease severity.
Giannakopoulos and Krilis [2013] show that change to β2GP1’s protein domains, notably the V domain, exposes an epitope that binds aβ2GP1. This change may be caused by oxidative stress following viral infection in which case it follows it is may also be the case following vaccination from the mRNA attempting to transcribe the structural spike protein domain within healthy cells. In addition, there is the risk of the nanocarrier being detected by circulating aPL IgG/IgM antibodies in the humoral system. If you hear the terms leaky or leakage used about ‘vaccines’, it refers to leakage from the nanocarrier. This would represent one or more of the two-hits and would cause subsequent binding to β2GP1 in healthy cells.
This is in additional ADE-risk to that of non-aPL IgG/IgM isotypes binding at ineffectively low levels to neutralisation escape variants.
The Two-Hit Theory.
The ‘two-hit theory’ that is relevant to PEG-binding antibodies also applies to phospholipid-binding antibodies. In a treatment-naive individual the first hit would be either viral infection or administering an antiviral. Each subsequent dosage of the antiviral would provide a second and subsequent hit.
Where healthcare bodies and/or pharmaceutical companies are talking about third or even fourth doses, or six-monthly boosters, this would create a sustained increase in aPL antibody activation and proliferation. In simple terms, the fewer the ‘hits’ the better; the greater the number of hits, the higher the risk of autoimmunity and immunopathology.
Zuo 𝘦𝘵 𝘢𝘭. show in patients with moderate to high COVID-19 disease severity that the majority have high levels of aCL IgM and aPS/PT IgG/IgM isotypes. What is clear is the general absence of IgA isotype, which is to be expected as it is the dominant antibody class in the upper respiratory tract not the humoral system. This is why you do not target a pathogen that binds in the epithelial upper respiratory tract with antibodies circulating in the endothelial humoral system.
Conclusions.
You would want to ensure that all the risks – known, established risks – associated with using phospholipid as an adjuvant in a treatment candidate had been fully explored. This has not happened.
- Antiphospholipid antibodies can be stimulated following SARS-CoV-2 infection and ‘vaccination’.
- ARDS may be being triggered by APS and not COVID-19, meaning that the ‘vaccine’ is causing the same disease as the virus it is supposed to treat.
- Antiphospholipid antibody levels should be a default check for those hospitalised with COVID-19 disease severity, firstly to isolate the underlying cause and secondly as it can be used as a biomarker of disease progression. As Tung et al. conclude; “Effective screening strategies for aPLs in the early phase of COVID-19 are highly desirable in order to stratify patients into low- and high-risk groups. Preventive measures can be initiated in high-risk groups to reduce thromboembolic complications and mortality in COVID-19 patients.” [our emboldening for emphasis]
- ARDS connects COVID-19 and APS/CAPS as the presenting symptoms and pathophysiology are similar. Tung et al. again; “The myriad of clinical presentations seen in patients with severe COVID-19 bear uncanny resemblances to those of patients with CAPS.”
- Investigation that should have done before mRNA experimental vaccines were rushed out before completion of full clinical trials was either not done or was ignored. In respect of both BNT126b2 and mRNA-1273, those trials that were undertaken were fundamentally flawed through excluded data, as we explained in April.
- The question must be explored as to whether vaccine-induced antiphospholipid antibodies are leading to ARDS that is being labelled as COVID-19.